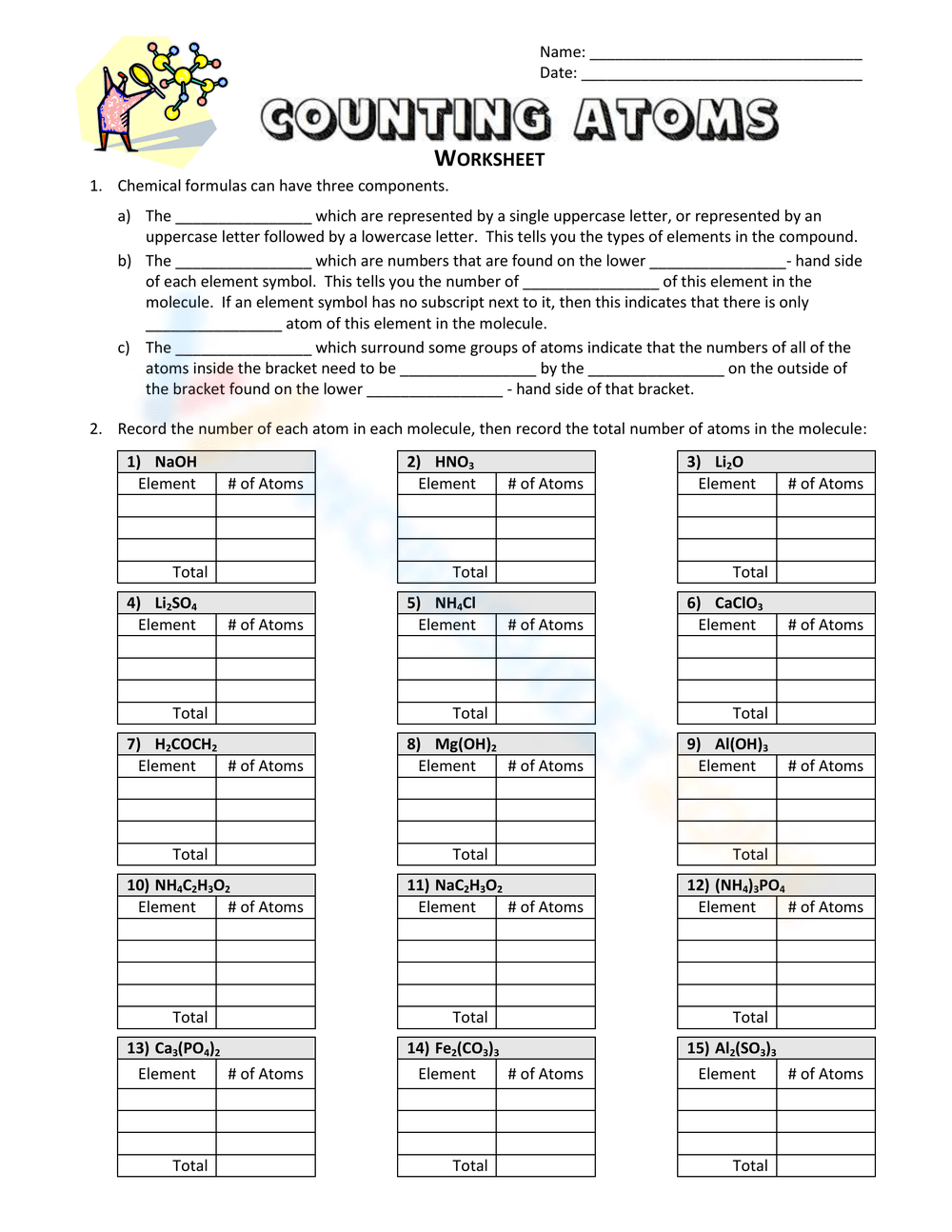
Master Counting Atoms with Worksheet 2: Easy Guide

When diving into the world of chemistry, one of the first skills you must master is counting atoms within chemical formulas. This isn't just about simple addition; it involves understanding molecular structures, recognizing subscripts and coefficients, and identifying types of atoms. In this guide, we'll explore how to effectively use Worksheet 2 to practice and improve your atom-counting abilities, ensuring that you're well-prepared for more complex chemical calculations.
Understanding Chemical Formulas

Before diving into the worksheet, let’s revisit the basics:
- Atoms are the smallest unit of an element.
- Chemical formulas describe the composition of compounds using element symbols and numbers.
- The subscript indicates how many atoms of each element are in one molecule.
- Chemical reactions might use coefficients to show how many molecules or formula units are involved.
How to Approach Worksheet 2

Worksheet 2 is designed to challenge and reinforce your atom-counting skills:
- Identify the Elements: Start by identifying every element in the given formula.
- Count the Atoms:
- If the element has no subscript, it’s one atom.
- If there’s a subscript, that’s the number of atoms.
- When there are parentheses, multiply all the atoms inside by the subscript outside.
- Consider Coefficients: If there are coefficients, multiply the whole count by this number.
- Summarize: Write down the total number of each element’s atoms.
🔬 Note: Be careful with the order of operations when dealing with nested parentheses or coefficients. Work from inside out.
Practical Example with Table
| Formula | Element | Number of Atoms |
|---|---|---|
| H2O | H | 2 |
| H2O | O | 1 |
| 2MgCl2 | Mg | 2 |
| 2MgCl2 | Cl | 4 |

Common Pitfalls

- Subscripts vs. Coefficients: Subscripts apply to each molecule, while coefficients apply to all molecules in the formula.
- Parentheses: Often forgotten, parentheses distribute the following subscript.
- Element Ambiguities: Watch out for similar symbols like ‘C’ for carbon and ‘Cu’ for copper.
📝 Note: If you're unsure, practice writing out the atom count for each element in the formula before tallying them up.
Making the Most of Worksheet 2

Here are some tips to maximize your learning:
- Vary the Complexity: Start with simpler formulas and progress to more complex ones.
- Check Your Work: After completing the worksheet, recheck each formula for errors.
- Practice Regularly: Like any skill, regular practice with different formulas can hone your accuracy.
- Peer Learning: Working through the worksheet with others can help catch overlooked mistakes and provides a different perspective.
In sum, mastering the art of counting atoms using Worksheet 2 involves a combination of understanding chemical notation, careful calculation, and consistent practice. By engaging with these exercises, you'll not only improve your technical skills but also build a solid foundation for more advanced chemistry. The ability to swiftly count atoms is crucial for understanding chemical reactions, balancing equations, and even delving into the exciting world of stoichiometry.
Why is it important to know how to count atoms in chemical formulas?

+
Understanding atom counts is foundational for chemistry as it allows you to calculate ratios in chemical reactions, predict reaction outcomes, and perform stoichiometry calculations.
What should I do if I struggle with complex formulas?

+
Start with simpler formulas and work your way up. Use mnemonics, break down formulas step-by-step, or seek explanations from your instructor or resources.
Can Worksheet 2 be used for self-study?

+
Yes, it’s designed to help reinforce atom-counting skills, making it excellent for self-paced learning or homework practice.
How can I double-check my answers?

+
You can use online atom counters, verify with a partner, or refer to answer keys if available. A second look can often reveal overlooked errors.