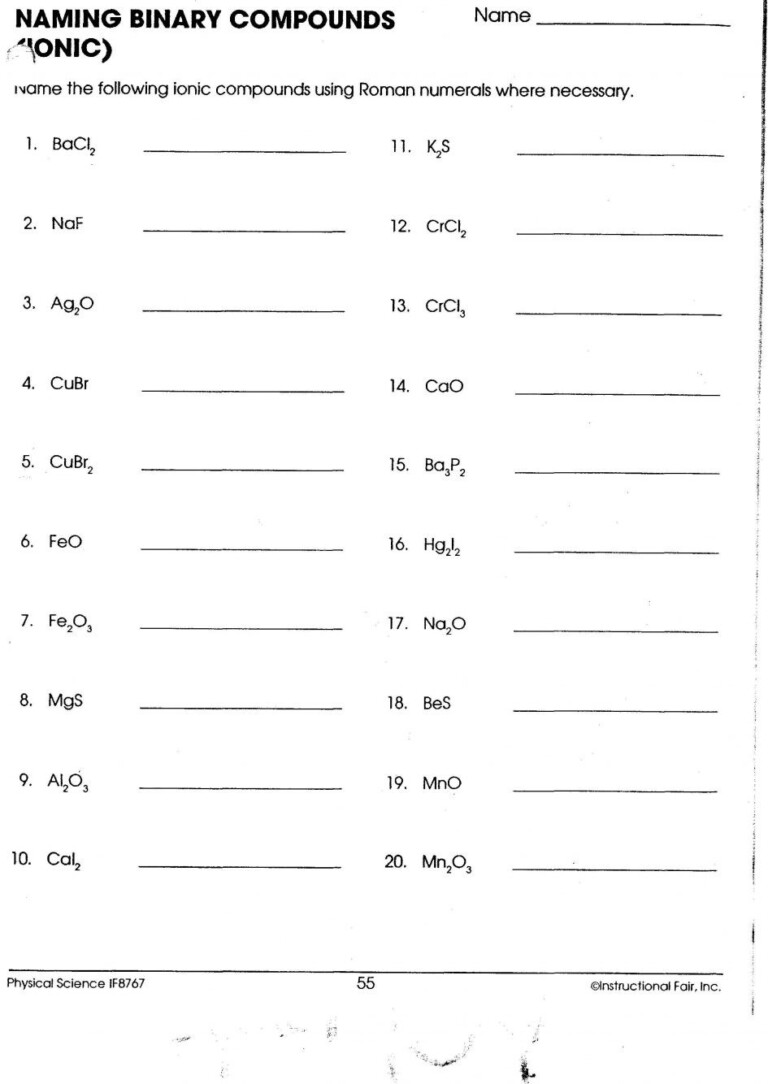
Counting Atoms Worksheet Answers: Master Chemistry Basics
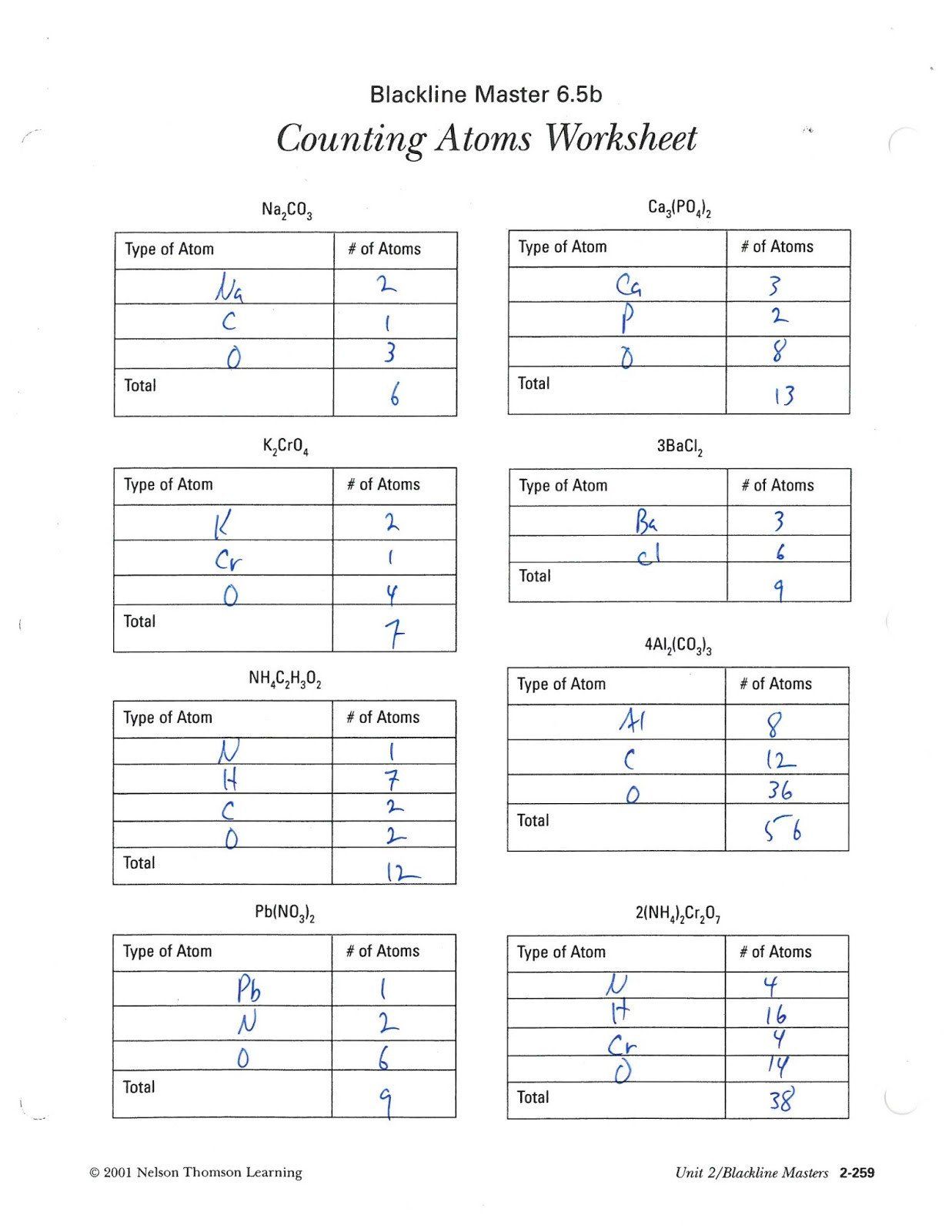
Embarking on a journey through the molecular world requires a fundamental understanding of its basic building blocks - atoms. Chemistry, often perceived as a complex subject, can indeed become an enthralling exploration once you master counting atoms. This comprehensive guide offers insights, practical worksheets, and detailed answers to help you effortlessly count atoms in chemical compounds. Whether you are a high school student or an enthusiast looking to brush up your chemistry skills, this blog post will clarify and demystify the process for you.
Why Counting Atoms Matters
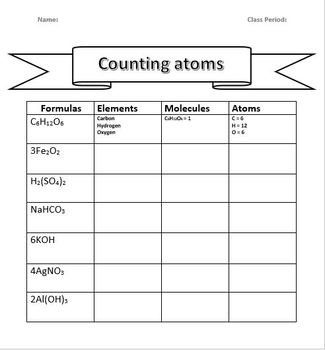
Before we dive into the how-tos, let’s quickly address why learning to count atoms is crucial:
- Chemical Reactions: Understanding how many atoms are in reactants helps in predicting the products of a chemical reaction.
- Stoichiometry: A precise count is essential for correct mole calculations and balancing equations.
- Structure and Bonding: The number of atoms impacts molecular structure, influencing the properties and behavior of substances.

Basic Principles for Counting Atoms

Here are the foundational steps to count atoms in a chemical compound:
- Identify the elements: Scan the compound for each unique element symbol.
- Check subscripts: Subscripts indicate the number of atoms for each element within parentheses or in a formula.
- Multiply by coefficients: If the compound is part of a larger formula, multiply the atoms by the coefficient preceding it.
- Handle complex molecules: For compounds with parentheses, distribute the subscript outside the parenthesis to all elements inside.
- Sum up: Combine all counts to get the total number of atoms.
🔬 Note: Remember, diatomic elements (like O2) count as two atoms per molecule.
Counting Atoms Worksheet

Let’s practice with some real-world examples:
- Calculate the number of atoms in glucose, C6H12O6.
- Count atoms in calcium carbonate, CaCO3.
- Determine atoms in sodium bicarbonate, NaHCO3.
- Analyze the number of atoms in ethanol, C2H5OH.
Example 1: Glucose

Glucose formula: C6H12O6
- Carbon: 6 atoms
- Hydrogen: 12 atoms
- Oxygen: 6 atoms
🔬 Note: If the compound had a coefficient, you would multiply the atoms by that number.
Example 2: Calcium Carbonate

Calcium carbonate formula: CaCO3
- Calcium: 1 atom
- Carbon: 1 atom
- Oxygen: 3 atoms
Example 3: Sodium Bicarbonate

Sodium bicarbonate formula: NaHCO3
- Sodium: 1 atom
- Hydrogen: 1 atom
- Carbon: 1 atom
- Oxygen: 3 atoms
Example 4: Ethanol

Ethanol formula: C2H5OH
- Carbon: 2 atoms
- Hydrogen: 6 atoms (5 from C2H5 + 1 from OH)
- Oxygen: 1 atom
Additional Tips for Counting Atoms

- Be mindful of hydrates - compounds containing water molecules, e.g., CuSO4·5H2O.
- Remember, if a compound is part of a polyatomic ion, the subscript outside the ion affects all atoms within it.
- Chemically similar compounds might have different atom counts, so always verify.
In closing, mastering the skill of counting atoms not only deepens your understanding of chemical composition but also enhances your ability to predict reactions, interpret chemical formulae, and comprehend the vast universe of molecules. The ability to count atoms accurately is the key to unlocking the mysteries of chemistry. We’ve explored the principles, provided practical examples, and highlighted common pitfalls to avoid. Keep practicing, and soon, you’ll count atoms with ease, making chemistry an enjoyable and insightful subject to explore.
What does the subscript indicate in a chemical formula?

+
The subscript in a chemical formula indicates the number of atoms of that element in the compound.
How do you count atoms in compounds with parentheses?

+
Distribute the subscript outside the parentheses to all atoms within. If no subscript, count each element as per the formula inside the parentheses.
Can the same compound have a different number of atoms?

+
Yes, if the compound is part of a different chemical reaction or if it’s involved in a hydrate or other ionic bonding scenarios.