Ionic Transition Metals Nomenclature Worksheet Formulas
Understanding the nomenclature of ionic compounds involving transition metals can be a challenging yet fascinating part of chemistry education. Transition metals, due to their multiple oxidation states, require a special approach when naming their compounds. This blog post will walk you through the fundamentals of naming ionic compounds with transition metals, provide you with a useful worksheet, and offer key insights into mastering this area of chemical nomenclature.
Naming Basics for Ionic Compounds with Transition Metals
The key to successfully naming compounds that include transition metals lies in recognizing their variable charges. Here’s how you can master it:
- Identify the Transition Metal: Transition metals are elements from groups 3 to 12 on the periodic table.
- Determine the Charge: Since transition metals can exhibit multiple charges, you’ll need to ascertain the metal’s charge. This can be done by:
- Looking at the known oxidation states of the metal.
- Balancing the compound’s overall charge with the known charges of the anions involved.
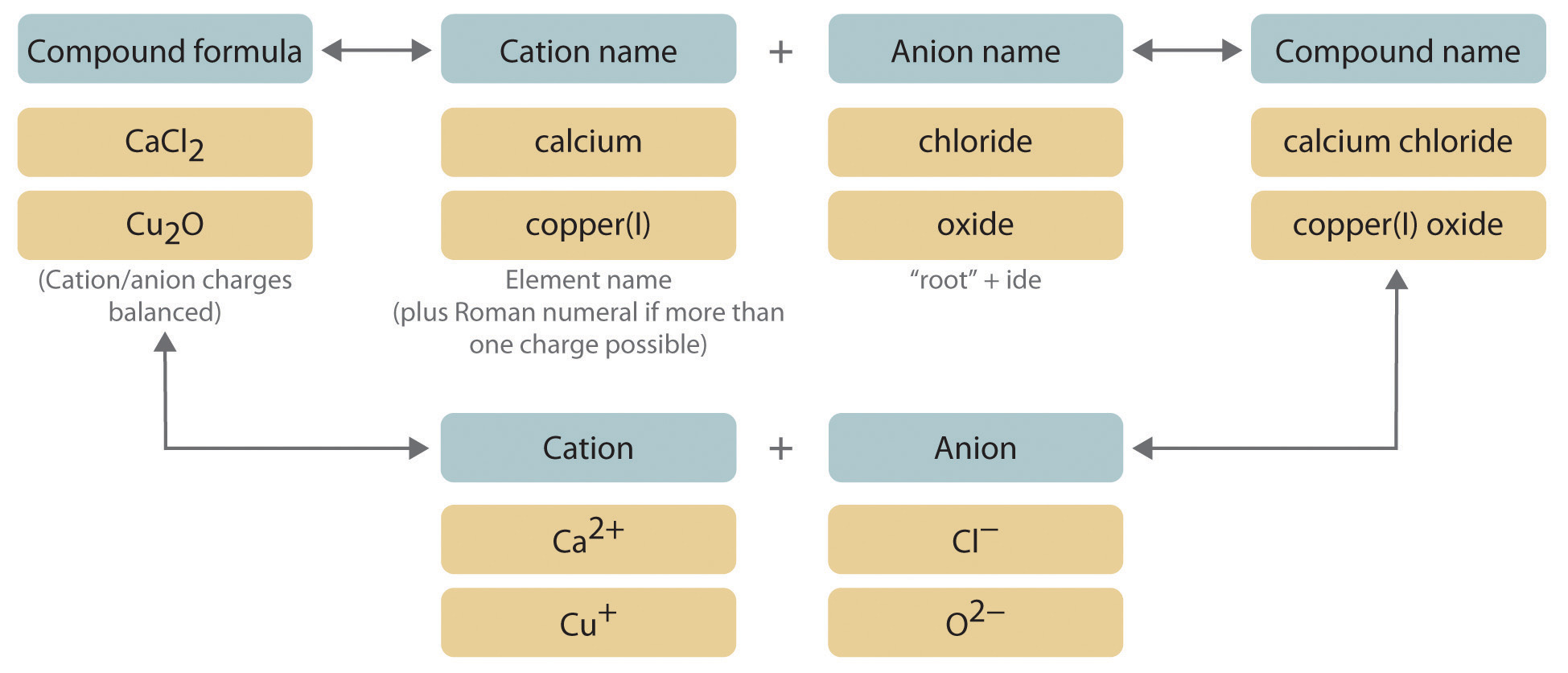
- Naming Convention: Use Roman numerals in parentheses immediately following the metal’s name to indicate its oxidation state. For example:
- Iron(II) chloride
- Copper(I) oxide
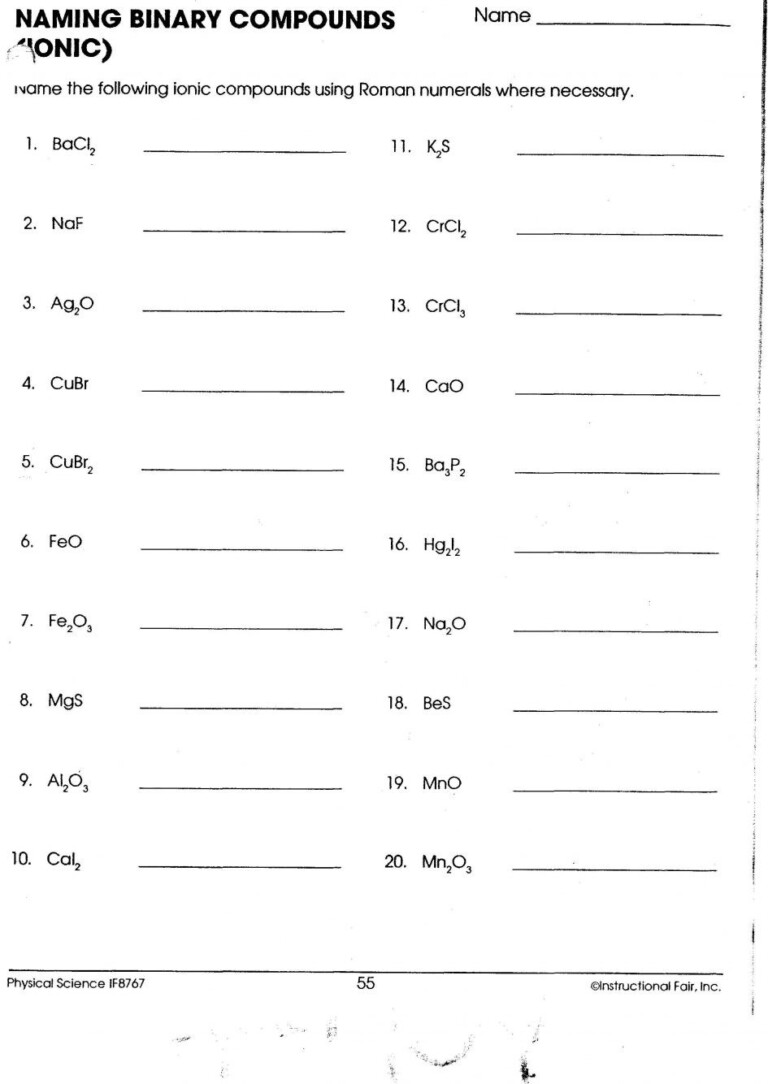
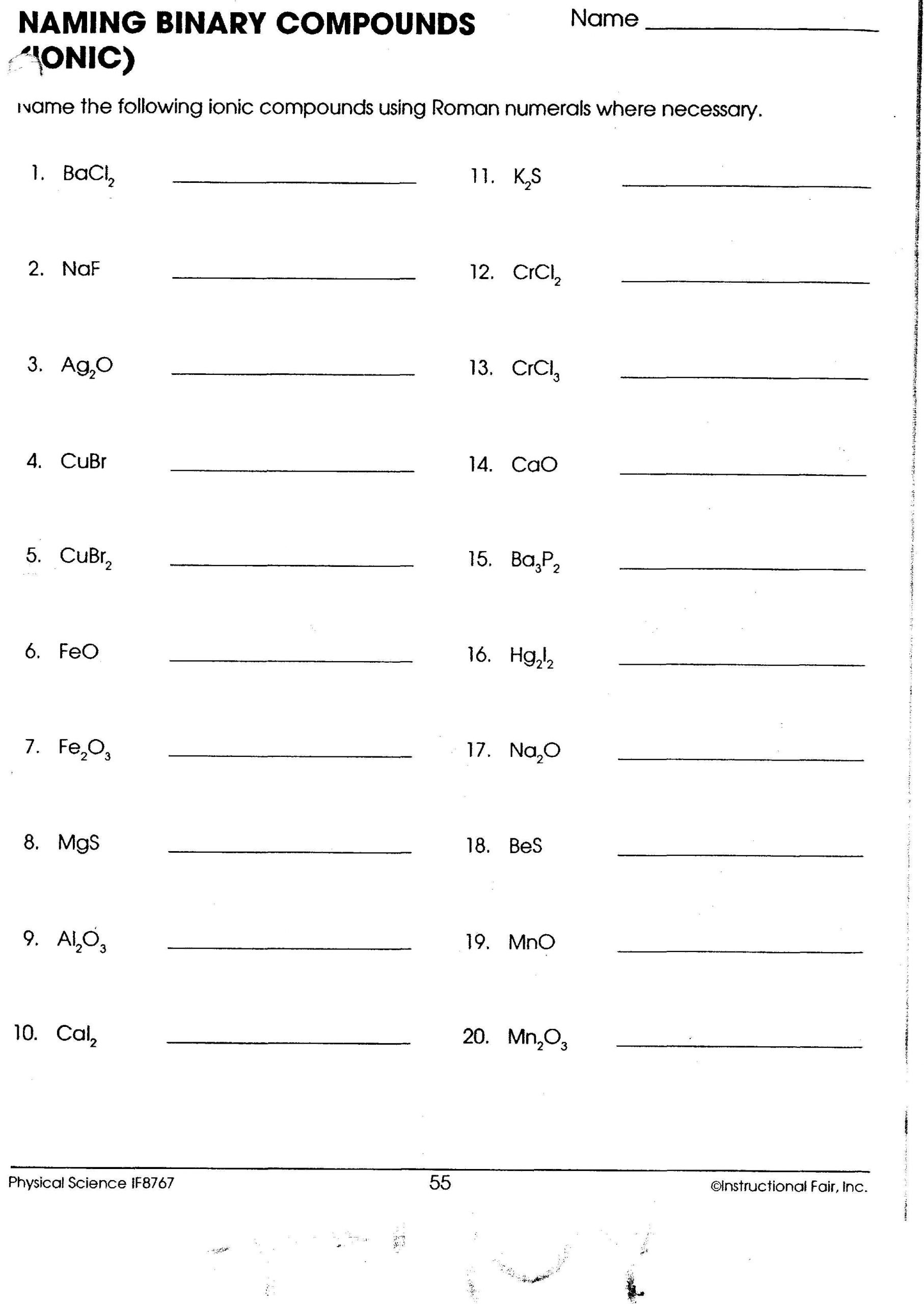
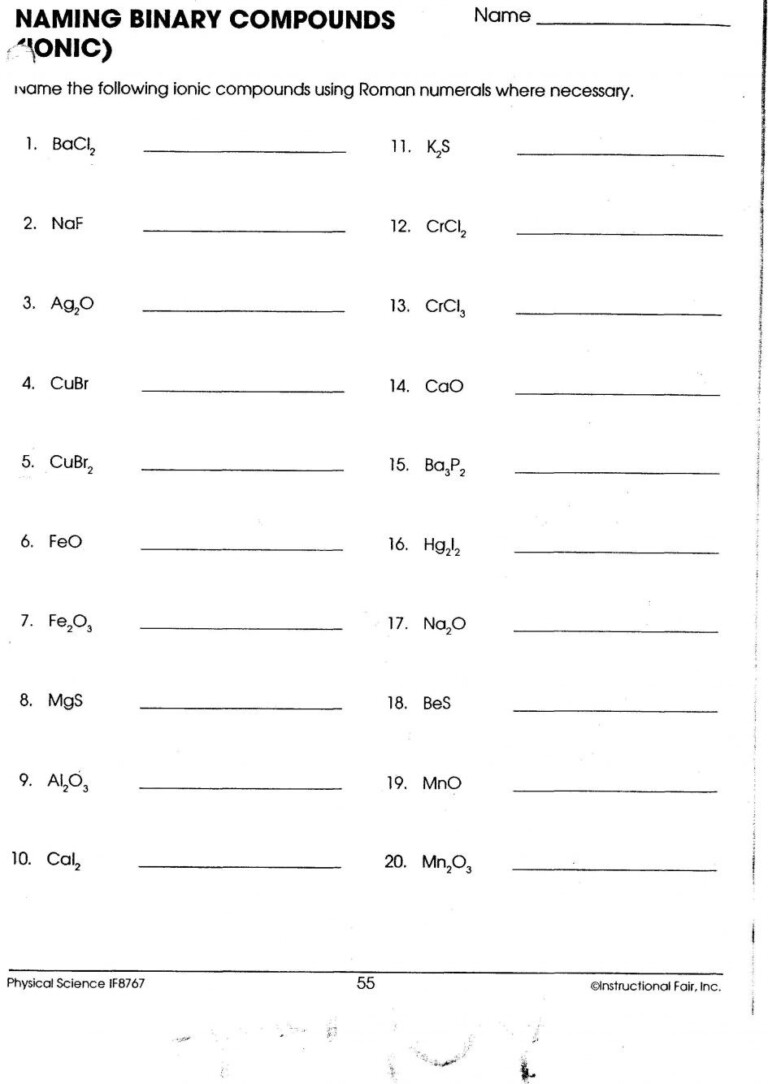
Worksheet for Practice

To solidify your understanding of naming transition metal ionic compounds, here’s a comprehensive worksheet you can use for practice:
| Formula | Name |
|---|---|
| FeCl2 | |
| CuO | |
| V2O5 | |
| MnCl3 | |
| TiO2 | |
| Fe2O3 | |
| CoF3 | |
| ZnCl2 |

💡 Note: While this worksheet is for practice, remember that the charge of some transition metals is fixed in certain compounds (e.g., Zn is always +2 in zinc compounds).
Strategies for Naming Ionic Compounds with Transition Metals
To ensure you’re correctly naming these compounds:
- Use the Periodic Table: Always have a periodic table at hand to quickly check common oxidation states.
- Charge Balance: Make sure the sum of positive and negative charges equals zero. This will help you determine the correct oxidation state for the metal.
- Common Mistakes: Be cautious with metals like copper and iron, which can have two common charges. Double-check the charges when in doubt.
Advanced Nomenclature Techniques

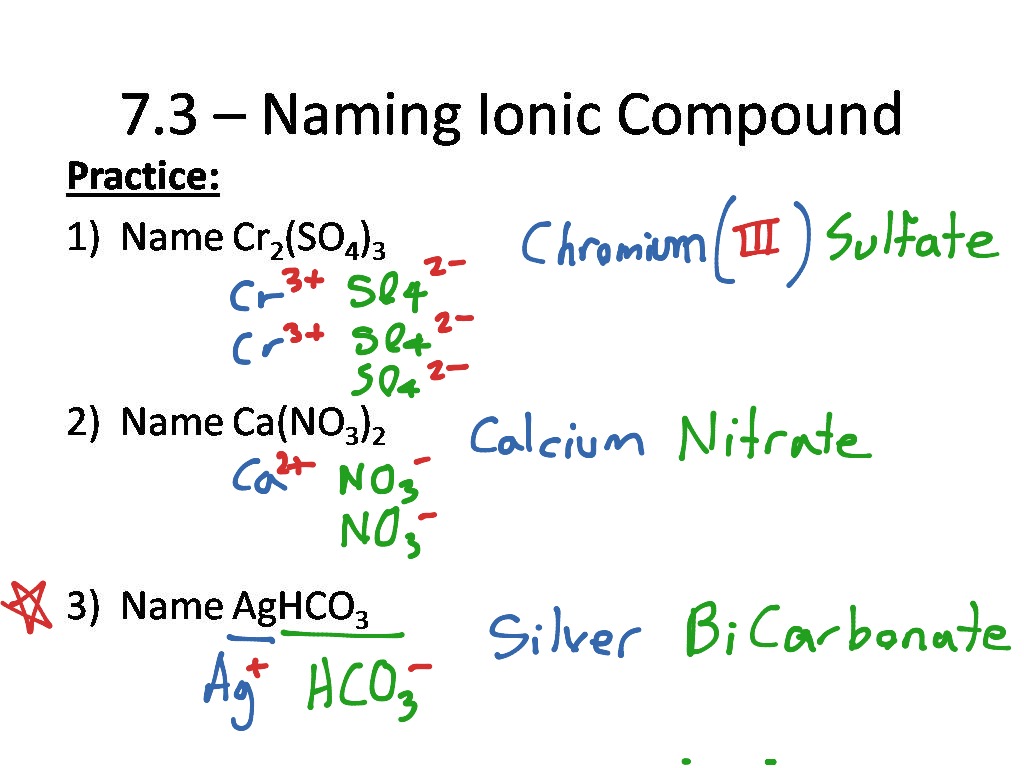
While most compounds are named using the Roman numeral system, there are exceptions and advanced rules to consider:
- Complex Ions: Compounds involving polyatomic ions require a different approach. Ensure you recognize and name these ions correctly.
- Stock System vs. Traditional Naming: Understand that while the Stock system (using Roman numerals) is preferred, traditional naming like ferric chloride (for FeCl3) still appears in some contexts.
- Magnetic and Color Properties: Transition metal compounds can have unique magnetic and color properties, which might be hinted at by their charge states.
In summary, mastering the nomenclature of ionic compounds with transition metals involves recognizing their variable charges, applying the Stock naming system, and practicing with real-world examples. With dedication and practice, you'll become proficient in interpreting and creating accurate names for these compounds, enhancing both your theoretical knowledge and practical application in chemistry.
Why do transition metals have variable charges?
+
Transition metals have incomplete d-orbitals which can participate in chemical bonding, leading to the ability to form ions with different charges.
Can I use traditional names instead of Roman numerals for transition metal compounds?
+
Yes, while the Stock system is now standard, traditional names like ferrous (Fe2+) and ferric (Fe3+) are still recognized in certain contexts.
How do I know the charge of a transition metal in a compound?
+
You can infer the metal’s charge by balancing the overall charge of the compound with known charges of anions, or by consulting a periodic table or a chemical dictionary for common oxidation states.