Chemistry Stoichiometry Worksheet: Master Essential Calculations Easily
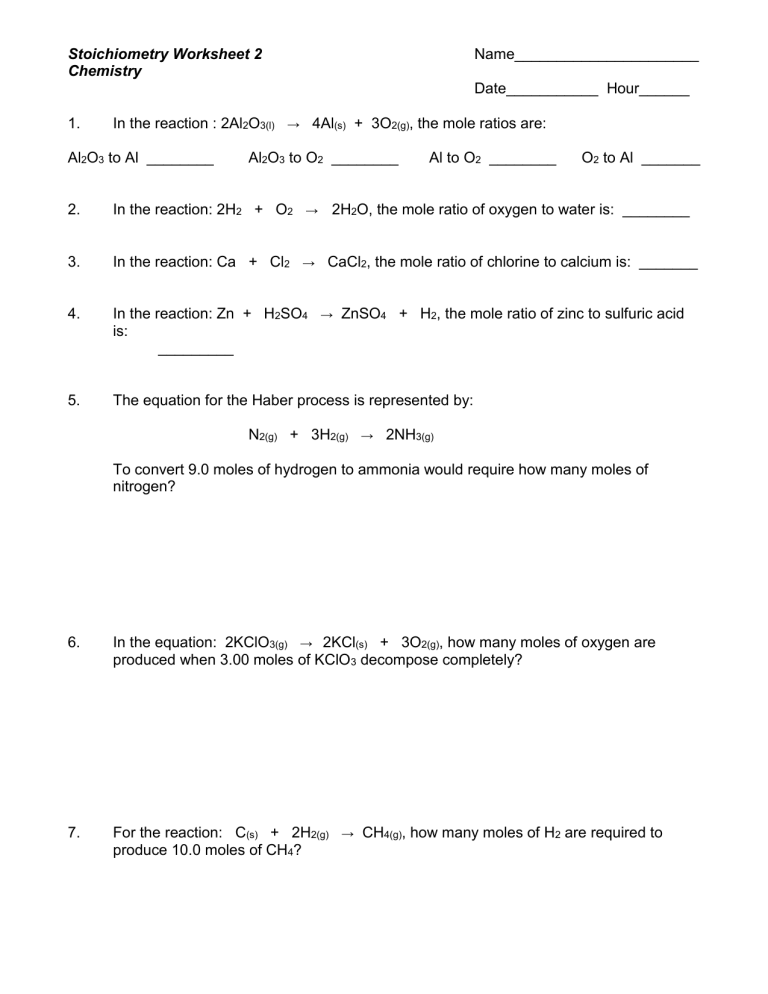
In the realm of chemistry, mastering stoichiometry is like unlocking a treasure trove of understanding reactions at the molecular level. Imagine being able to predict the amount of substances needed or produced in chemical reactions with precision. Stoichiometry, often considered the math of chemistry, provides this insight, and today, we'll journey through its core principles and calculations using a well-structured worksheet.
Understanding Stoichiometry

Stoichiometry involves calculating the quantities of reactants and products in chemical reactions using balanced chemical equations. Here’s a quick rundown:
- Balanced chemical equation: A prerequisite for accurate stoichiometry, ensuring atoms are conserved.
- Molar ratio: Derived from the coefficients in the balanced equation, it’s the ratio of moles between reactants and products.
- Molecular weight (Molar mass): This tells us how much mass a mole of substance has, crucial for converting between mass and moles.
Let's Dive Into Some Examples:

Balancing the Equation

First, we need to balance the chemical equation. For instance, consider the combustion of propane:
C3H8 + O2 -> CO2 + H2O
Balancing this gives us:
C3H8 + 5O2 -> 3CO2 + 4H2O
Stoichiometric Calculations

- Converting Mass to Moles: Use the molecular weight to convert the given mass of a substance into moles.
- Using Molar Ratios: Apply the balanced equation's coefficients to calculate the moles of another substance involved.
- Converting Moles Back to Mass: Convert the moles calculated to grams using the molecular weight again.
Here's an Example:
If we burn 24 g of C3H8, how many grams of O2 are required?
| Step | Calculation | Result |
| Convert mass to moles of C3H8 | 24 g / 44 g/mol | 0.545 mol |
| Use Molar Ratio to find moles of O2 | 0.545 mol C3H8 * 5/1 | 2.725 mol O2 |
| Convert moles of O2 to grams | 2.725 mol * 32 g/mol | 87.2 g O2 |

⚗️ Note: Always ensure that the chemical equation is balanced before starting calculations to maintain stoichiometric accuracy.
More Stoichiometry Exercises

Let’s explore other stoichiometric problems:
- Determine the amount of product from given reactant:
- Find limiting reagent:
- Percent Yield:
If you have 20.0 g of Na, how many grams of NaCl can be produced in the reaction with Cl2?
Given 15.0 g of NaOH and 10.0 g of HCl, which is the limiting reagent and how much NaCl can be produced?
If you obtain 12.0 g of NaCl from the reaction of 15.0 g of NaOH with excess HCl, what is the percent yield?
Practical Tips for Stoichiometry

- Always double-check the balancing of your equation.
- Use dimensional analysis (units canceling out) for clarity in conversion steps.
- Know the atomic weights and molecular weights for quick calculations.
📝 Note: Practice with real-world problems to get comfortable with stoichiometric calculations.
Real-World Applications of Stoichiometry

Stoichiometry isn’t just an academic exercise; it’s crucial in various fields:
- Pharmacology: Determining drug dosages.
- Environmental Science: Calculating pollution from emissions.
- Food Industry: Ensuring the right mixture of ingredients for taste and safety.
As we wind down our exploration into the world of stoichiometry, it's important to recognize the power this knowledge wields in understanding and controlling the natural world at a microscopic level. Stoichiometry not only helps us comprehend how substances interact but also enables us to manipulate these interactions for practical benefits. The calculations might seem intricate at first, but with the structured approach provided by worksheets, your journey into mastering stoichiometry will be streamlined and insightful. Whether you're aiming to balance equations, calculate theoretical yields, or explore the limits of reactions, the skills you develop in stoichiometry will serve as a cornerstone for your future endeavors in chemistry.
Embarking on this journey requires patience and practice, but the rewards are immense. Understanding the dance of atoms and molecules through stoichiometry opens up new worlds of scientific and industrial applications, empowering us to create, innovate, and solve some of the world's most pressing problems.
What is the most important step in stoichiometric calculations?
+
The most crucial step is ensuring the chemical equation is balanced. This provides the correct molar ratios between reactants and products necessary for accurate calculations.
Can you do stoichiometric calculations without the molecular weight?

+
No, molecular weight (or molar mass) is essential for converting between mass and moles. Without it, the stoichiometric relationships would not hold.
How can stoichiometry help in environmental protection?

+
Stoichiometry enables scientists to predict and control the amount of pollutants produced in industrial processes, facilitating better environmental management and sustainability efforts.