Molarity Mastery: Chemistry Worksheet Answers Revealed

Understanding molarity is a fundamental concept in chemistry that involves measuring the concentration of a solute in a solution. It's a measure of the amount of substance (in moles) dissolved per liter of solution, and it's expressed with the units mol/L or M. Molarity is vital in various chemistry-related fields such as pharmaceuticals, environmental science, and even in cooking, where precision in measurements is crucial for consistent results.
What is Molarity?
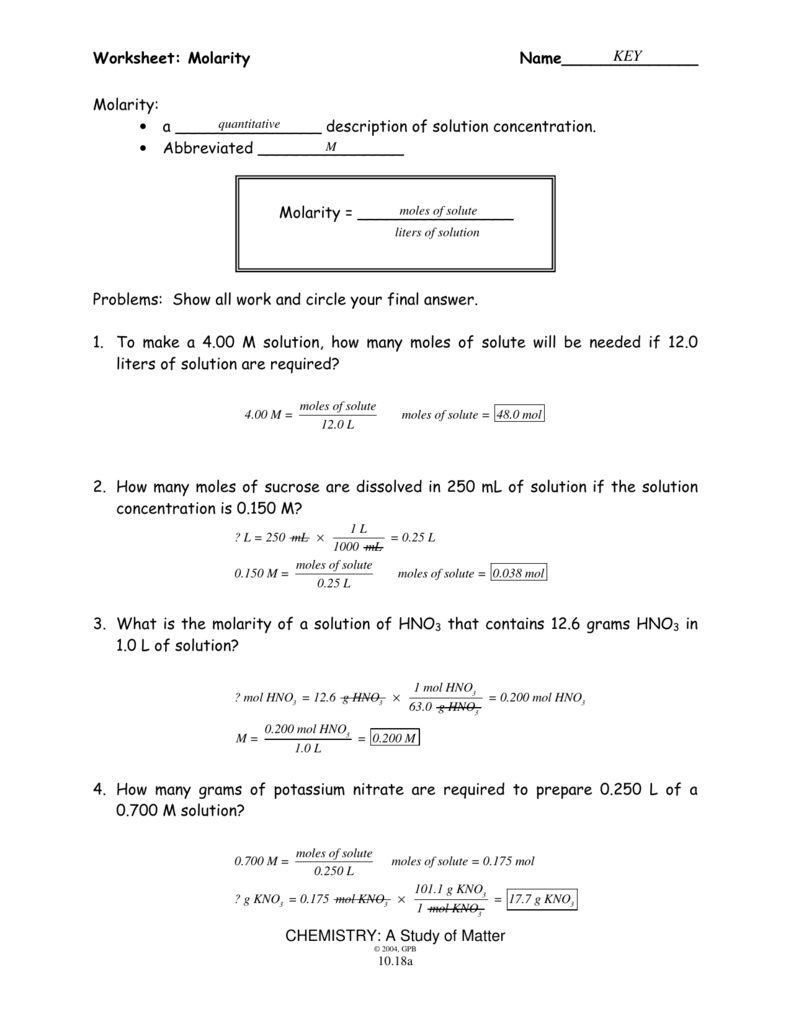
Molarity (M), sometimes referred to as molar concentration, is defined as the number of moles of solute per liter of solution. Here’s how you calculate it:
- Step 1: Determine the moles of solute (n): n = mass of solute / molar mass
- Step 2: Determine the volume of the solution (V) in liters
- Step 3: Apply the formula for molarity: M = n / V
Now, let's dive into an example for clarity:
Example Calculation

Suppose we have a solution made by dissolving 5.85 grams of sodium chloride (NaCl) in 250 mL of water. What is the molarity?
- The molar mass of NaCl is approximately 58.44 g/mol.
- The volume of the solution must be converted to liters: 250 mL = 0.250 L
To find the moles of NaCl:
moles = mass / molar mass = 5.85 g / 58.44 g/mol ≈ 0.100 mol
Now, calculate the molarity:
M = n / V = 0.100 mol / 0.250 L = 0.400 M
How to Use Molarity in Calculations

Molarity plays a crucial role in:
- Preparing solutions with a specific concentration
- Diluting solutions
- Reacting chemicals in stoichiometric calculations
Here's how to prepare a 1.5 M solution of glucose (C6H12O6) from its solid form:
- Weigh out the necessary mass of solute based on the molar mass of glucose (180.16 g/mol).
- Add water up to the desired volume (adjust for the final volume if glucose dissolves in less water).
⚠️ Note: Always mix well to ensure even distribution of the solute.
Worksheet Answers Revealed

Let’s explore some common worksheet problems related to molarity:
Question 1: Molar Mass and Molarity
If you dissolve 6.30 grams of iron(III) nitrate [Fe(NO3)3] in 500 mL of water, what is the molarity?
- The molar mass of Fe(NO3)3 is 241.86 g/mol.
- Convert volume to liters: 500 mL = 0.500 L
- Calculate moles: moles = 6.30 g / 241.86 g/mol ≈ 0.0260 mol
- Calculate molarity: M = 0.0260 mol / 0.500 L = 0.0520 M
Question 2: Dilution Problems

You have 200 mL of 0.500 M sodium hydroxide (NaOH) solution. How much water do you need to add to create a 0.250 M solution?
| Initial Concentration (M1) | Initial Volume (V1) | Final Concentration (M2) | Final Volume (V2) |
|---|---|---|---|
| 0.500 M | 200 mL | 0.250 M | 400 mL |

- Use the dilution formula: M1V1 = M2V2
- Find V2: V2 = M1V1 / M2 = (0.500 M × 200 mL) / 0.250 M = 400 mL
- The volume of water to add is V2 - V1 = 400 mL - 200 mL = 200 mL
In sum, this blog has explored the intricacies of molarity, covering its definition, calculation methods, and practical applications. By following the steps outlined and understanding the worksheet solutions, you'll find that measuring concentrations becomes an artful science, pivotal for accurate experimentation and chemical analysis.
Why is molarity important in chemical calculations?

+
Molarity helps in quantifying the concentration of solutions, which is crucial for reaction stoichiometry, lab experiments, and controlling the strength of solutions in various applications.
Can the volume of the solution change after dissolving the solute?

+
Yes, dissolving solute can slightly change the volume. However, for most molarity calculations, we assume the volume change is negligible to keep the calculations straightforward.
What are the differences between molarity and molality?

+
Molarity is the concentration based on volume, whereas molality is based on mass (moles of solute per kilogram of solvent). Molality is not affected by temperature, unlike molarity.