Balancing Chemical Equations: Easy Answers for Students

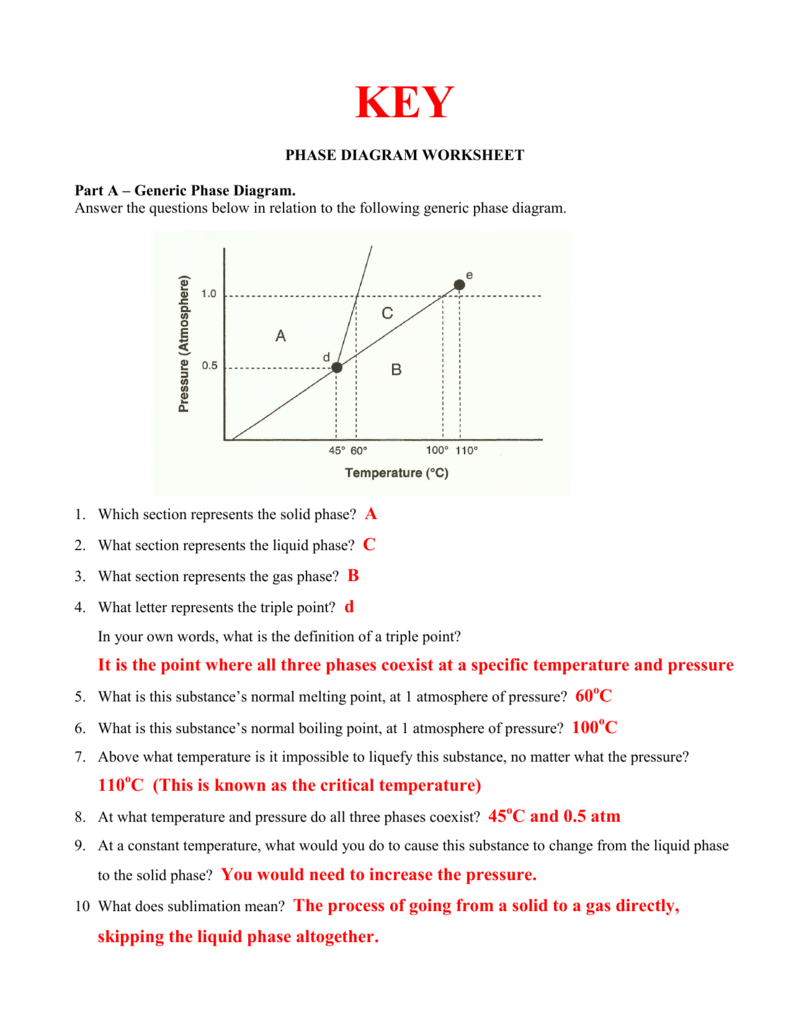
The Fundamentals of Chemical Equations

Every student of chemistry, whether you’re taking your first steps into the world of atoms and molecules or you’re a seasoned lab enthusiast, will encounter the task of balancing chemical equations. At its core, balancing equations is about understanding the conservation of mass - an essential concept in chemistry. When we talk about balancing chemical equations, we’re essentially ensuring that the number of atoms of each element involved in a chemical reaction remains constant from reactants to products.
Why Do We Need to Balance Equations?

The law of conservation of mass dictates that matter cannot be created or destroyed in a chemical reaction. Hence, a balanced equation is not just a scientific convention but a reflection of the reality of chemical changes:
- To comply with the Law of Conservation of Mass.
- To accurately predict the amount of product we can expect from given reactants.
- To calculate reaction yields and understand stoichiometry, which is crucial in both lab work and industrial applications.
The Steps to Balance a Chemical Equation

Balancing an equation isn’t random. Here are the systematic steps you can follow:
Step 1: Write the Unbalanced Equation
Begin with the skeletal equation, which shows the reactants and products involved. An example might be:
[ \text{CH}_4 + \text{O}_2 \rightarrow \text{CO}_2 + \text{H}_2\text{O} ]
Step 2: Balance the Atoms Apart from Hydrogen and Oxygen
Start with the most complex compound or element that isn’t hydrogen or oxygen. For our example:
⚗️ Note: This step can be skipped if all elements are balanced without it.
Step 3: Balance Hydrogen Atoms
Proceed to balance hydrogen. For example:
( \text{CH}_4 + \text{O}_2 \rightarrow \text{CO}_2 + 2\text{H}_2\text{O} )
Step 4: Balance Oxygen Atoms
Finally, balance oxygen. Sometimes, this requires revisiting the previously balanced atoms:
[ 2\text{CH}_4 + 2\text{O}_2 \rightarrow 2\text{CO}_2 + 4\text{H}_2\text{O} ]
Step 5: Double-Check Your Work
Count the atoms on both sides once more to ensure you have equal numbers:
| Element | Reactant Side | Product Side |
|---|---|---|
| Carbon © | 2 | 2 |
| Hydrogen (H) | 8 | 8 |
| Oxygen (O) | 6 | 6 |

Common Pitfalls and Tricks

Here are some tips to avoid common mistakes:
- Use the smallest whole number coefficients possible.
- Don’t change the subscripts of the compounds; only change the coefficients.
- If you end up with a fraction, multiply the whole equation by the denominator to clear it.
- Remember that polyatomic ions can often be balanced as a whole unit.
🧪 Note: Balancing polyatomic ions as units can simplify complex equations significantly.
Advanced Techniques for Complex Equations

Not all chemical reactions are straightforward. Here’s how to handle the complex ones:
- Inspection Method: Suitable for simple reactions but can become cumbersome with complex ones.
- Algebraic Method: For advanced students or when inspection fails, this method uses algebraic equations to balance chemical equations.
- Half-Reaction Method: Used for redox reactions where electrons are transferred between reactants.
Using Software and Calculators

While mastering the skill is beneficial, there are tools available for those moments when you need an answer quickly or for verification:
- Online calculators like those found on educational websites.
- Chemical equation balancers within scientific software.
- Mobile apps designed for chemistry students.
Practical Application

Understanding how to balance chemical equations is essential not just in the lab, but also in real-world applications:
- Environmental chemistry to predict reaction outputs.
- Pharmaceutical synthesis, where stoichiometry is key.
- Industrial processes like the Haber-Bosch process.
Tips for Success

Here’s how to enhance your learning and application:
- Practice with a variety of equations, from simple to complex.
- Understand the underlying chemical processes, not just the math.
- Use visual aids or models to conceptualize reactions.
🧐 Note: Remember, practice makes perfect. The more equations you balance, the more intuitive the process becomes.
Summary

In our exploration of balancing chemical equations, we’ve delved into the fundamental principles, the logical steps to achieve balance, common pitfalls to avoid, advanced techniques for complex reactions, and the practical applications of this skill. Understanding how to balance equations not only helps in maintaining the integrity of chemical reactions but also in understanding the stoichiometry of those reactions, predicting reaction outputs, and contributing to fields like environmental chemistry, pharmaceutical synthesis, and industrial processes. Keep in mind that while tools like calculators exist, the real mastery comes from understanding the chemistry behind the numbers.
Why is it important to balance chemical equations?

+
Balancing chemical equations is crucial because it reflects the law of conservation of mass, ensuring that the number of atoms of each element remains constant before and after the reaction, providing an accurate prediction of the products formed.
Can you balance any chemical equation?

+
Yes, any chemical equation can be balanced if it follows the laws of stoichiometry and conservation of mass. However, very complex equations might require more advanced methods to balance.
What if I get a fraction in my balanced equation?

+
Fractions are valid, but for simplicity, you should multiply the whole equation by the denominator of the fraction to get whole number coefficients.