5 Essential Answers for Phase Diagram Worksheets
Phase diagrams are crucial tools in materials science, chemistry, and physics, providing a visual representation of the physical states of substances under different conditions of temperature and pressure. These diagrams are not just academic; they have practical applications in industries ranging from metallurgy to pharmaceuticals. Understanding how to interpret and use phase diagrams can be challenging, particularly for students and newcomers to the field. Here, we address five essential aspects you need to know when working with phase diagrams, ensuring you can confidently navigate these complex charts.
Understanding the Basics of Phase Diagrams

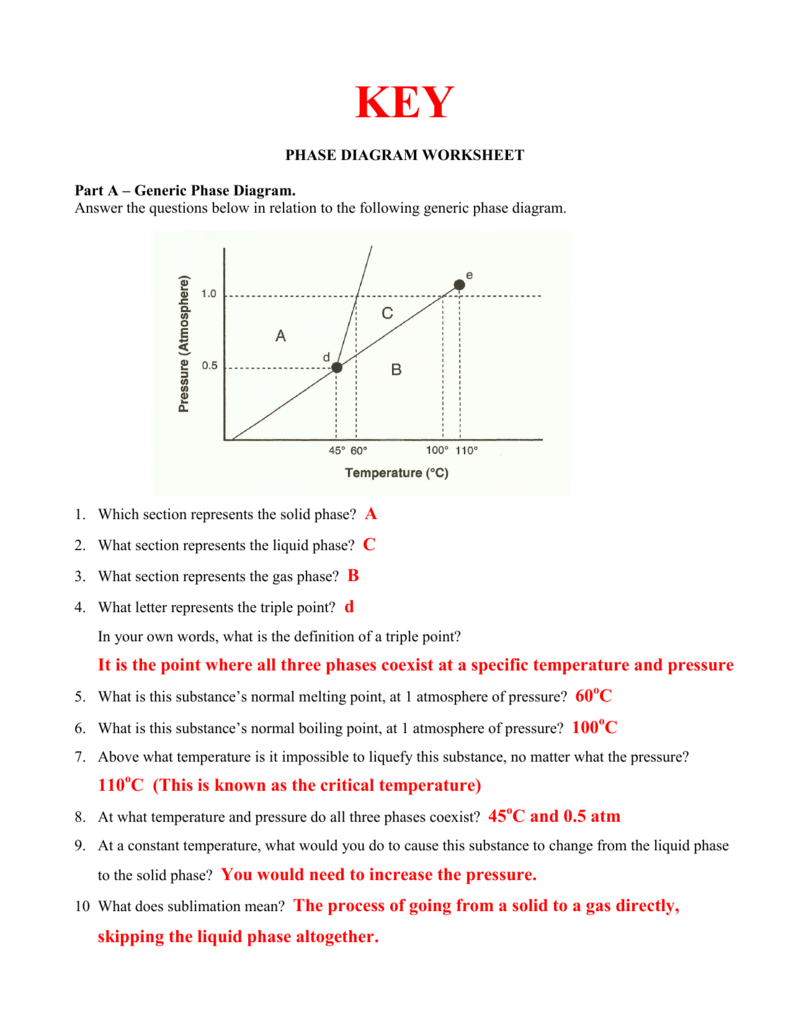
Before diving into the detailed analysis, one must grasp what a phase diagram actually is. A phase diagram maps out the phases of a substance (solid, liquid, gas) as conditions like temperature and pressure change:
- Pure Substances: For pure substances, a phase diagram typically shows three phases: solid, liquid, and vapor.
- Mixtures: Phase diagrams can also illustrate how mixtures of substances (like alloys or solutions) behave under varying conditions.

Key Components of a Phase Diagram

- Phase Boundaries: Lines that separate regions where different phases are stable.
- Critical Point: A point where the distinction between liquid and gas phases disappears.
- Triple Point: The unique condition where all three phases coexist in equilibrium.
📌 Note: Always check the conditions specified for a phase diagram. Some might use different axes or have additional considerations like pressure other than atmospheric.
Interpreting Phase Boundaries

The lines on a phase diagram aren't just decorative; they're markers of significant changes in state. Here's how you can interpret these boundaries:
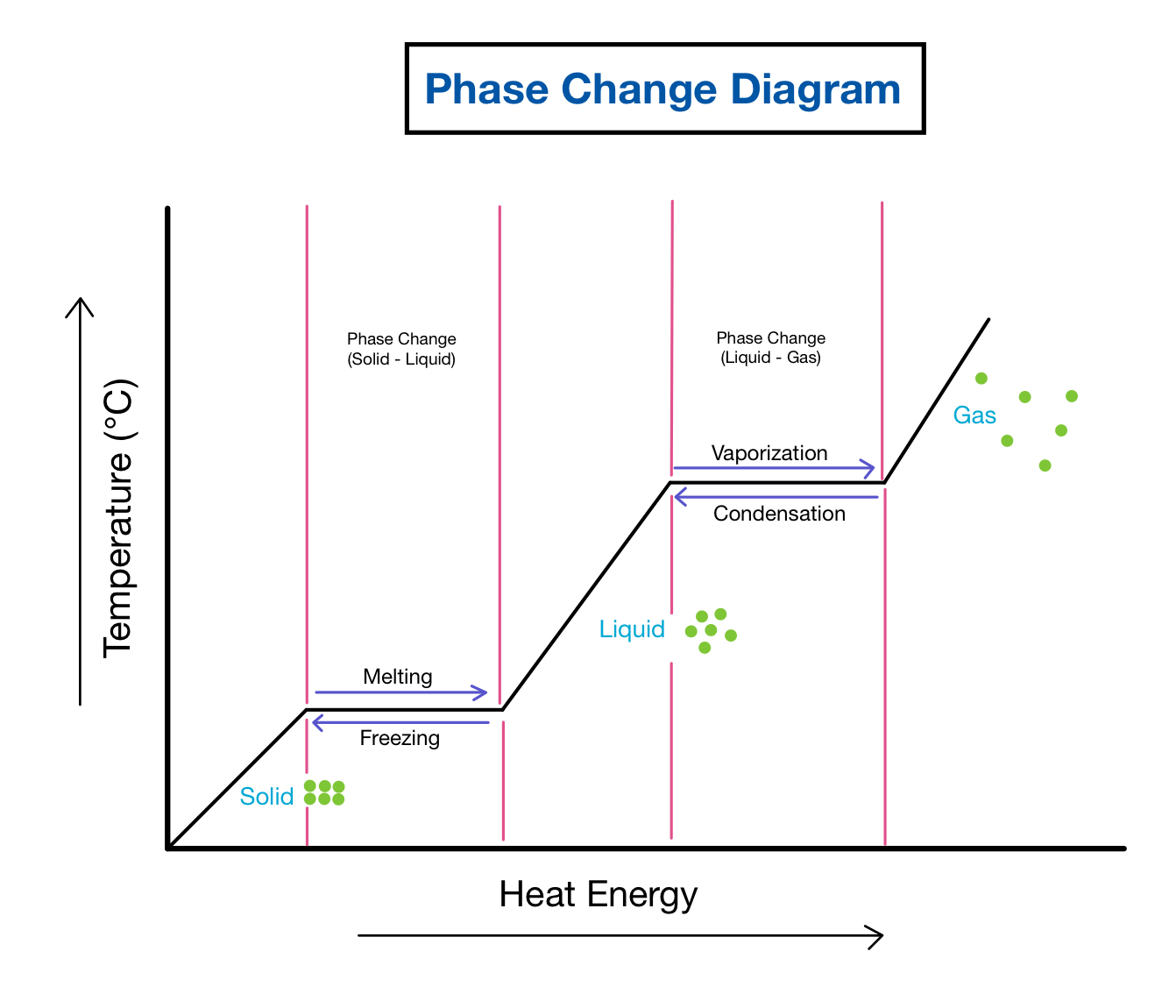
- Phase Transformation: Crossing a boundary indicates a phase change. For instance, moving from a solid to a liquid state across the melting line.
- Equilibrium: Points on these lines represent conditions where phases coexist in equilibrium.
Changes in State Explained

| Line | Change of State |
|---|---|
| Melting/Freezing Line | Conversion between solid and liquid |
| Vaporization/Condensation Line | Transition from liquid to gas or vice versa |
| Sublimation/Deposition Line | Direct change between solid and gas |

📌 Note: Some substances might not have all these lines, for instance, carbon dioxide doesn't have a liquid phase at atmospheric pressure, hence its sublimation line.
Analyzing the Triple Point and Critical Point

Two points on the phase diagram deserve special attention:
- Triple Point: Understanding this point helps you predict under what conditions a substance can exist in three different phases.
- Critical Point: This is where the differences between the liquid and gas phases vanish, leading to a supercritical fluid.
Significance of These Points

- The triple point gives insight into the behavior of substances at very low pressures and temperatures, which is useful in industries like cryogenics.
- The critical point is essential for processes involving supercritical extraction or fluid dynamics in chemical engineering.
📌 Note: Water's phase diagram is particularly famous because its triple point occurs at conditions close to normal atmospheric pressure, which is relatively rare.
Phase Diagram Applications in Industry

Phase diagrams aren't just academic curiosities; they have real-world applications:
- Alloy Development: Engineers use phase diagrams to design alloys with specific properties by manipulating phase stability and transitions.
- Chemical Processing: Understanding how substances behave under pressure can dictate process conditions in chemical manufacturing.
- Pharmaceutical Stability: Determining the stability of pharmaceuticals at different temperatures and pressures helps in drug formulation and storage.
Interpreting Phase Compositions

Phase diagrams can tell us more than just the state of a substance; they can help us understand the composition within phases:
- Phase Composition: For mixtures, phase diagrams can show what components are in what phase.
- Lever Rule: This rule allows one to calculate the percentage of each phase in a mixture at a given temperature.
In materials science, knowing how much of a substance is present in each phase can guide the development of new materials with tailored properties:
- Mechanical Properties: The strength or ductility of an alloy can be tuned by adjusting the phase composition.
- Electrical Conductivity: The conductivity of materials can change with the phase composition, which is critical for electronics.
📌 Note: While phase diagrams give an idea of phase composition, actual experimental verification is often necessary to account for kinetics and other real-world factors.
In summary, phase diagrams are a vital tool for understanding the behavior of substances under various conditions. They help in:
- Predicting how a substance will change phase as conditions vary.
- Determining the phase composition and stability of mixtures.
- Designing and controlling industrial processes where phase transitions are involved.
Mastering phase diagrams involves understanding their components, applications, and how to apply them to real-world scenarios. They are not just for academics; they're blueprints for industry and innovation. With this knowledge, you're equipped to tackle the complexities of materials behavior and make informed decisions in both laboratory and industrial settings.
What is the purpose of a phase diagram?

+
A phase diagram shows the stability of different phases of a substance under different temperature and pressure conditions. It’s used to predict phase transitions, understand material properties, and design processes in industries.
Can you explain the triple point?

+
The triple point is the unique set of conditions where a substance can exist in all three phases (solid, liquid, gas) simultaneously. This point is particularly important for substances like water, where it’s close to atmospheric pressure.
How are phase diagrams used in engineering?
+
In engineering, phase diagrams are critical for alloy design, predicting material behavior, optimizing process conditions in manufacturing, and in applications like supercritical fluid extraction where phase transitions are key.
What does the critical point signify in a phase diagram?

+
The critical point marks the end of the phase boundary between the liquid and gas phases. Beyond this point, the substance becomes a supercritical fluid, showing properties between those of a gas and a liquid.
How do you use the lever rule in phase diagrams?
+
The lever rule helps calculate the relative proportions of phases present in a mixture at a specific temperature. You draw a tie line between the phase boundaries and use the lengths of segments to find phase proportions.