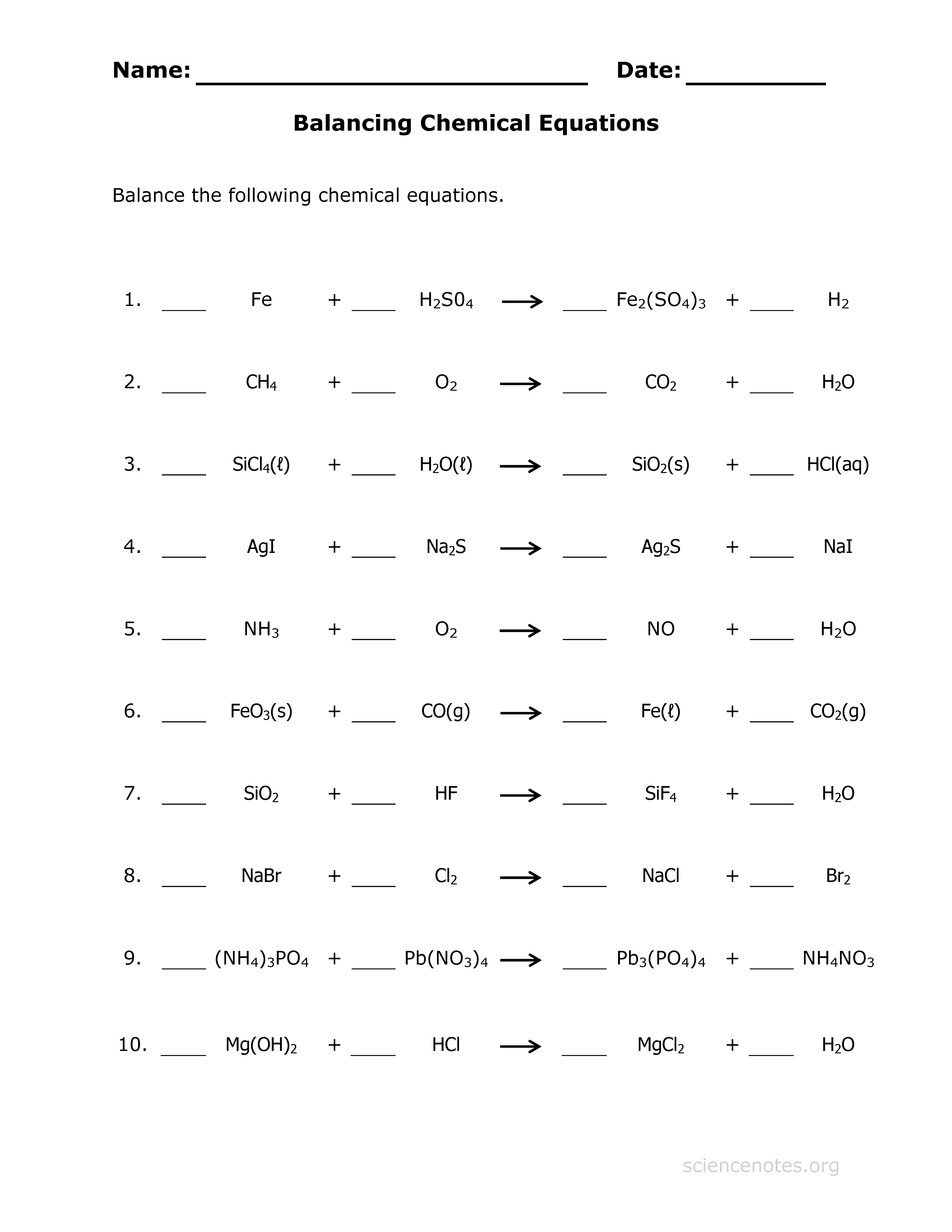
5 Easy Steps to Balance Chemical Equations Like a Pro

Balancing chemical equations is a fundamental skill in chemistry, essential for understanding chemical reactions and processes. Whether you're a student, an educator, or just curious about how the world works, knowing how to balance equations can give you a deeper insight into the nature of matter. This guide will take you through five easy steps to balance chemical equations, transforming you into a proficient problem solver in no time.
The Importance of Balancing Chemical Equations

Before diving into the steps, let's briefly explore why we balance chemical equations:
- Stoichiometry: Balancing ensures the law of conservation of mass, where the total number of atoms for each element on the reactants' side equals that on the products' side.
- Reaction Insight: It helps us understand how substances react and predict the outcomes of reactions.
- Chemical Calculations: Knowing the ratio of reactants to products is key for calculating amounts in reactions, often used in industry and research.
Step 1: Identify the Reactants and Products

First, let's look at an example equation:
CH4 + O2 → CO2 + H2O
In this equation:
- The reactants are methane (CH4) and oxygen (O2).
- The products are carbon dioxide (CO2) and water (H2O).
Step 2: Count the Atoms of Each Element

Now, count the atoms in the reactants and products:
| Element | Reactants | Products |
|---|---|---|
| Carbon (C) | 1 | 1 |
| Hydrogen (H) | 4 | 2 |
| Oxygen (O) | 2 | 3 |

⚠️ Note: This step helps to highlight the imbalances that need correction.
Step 3: Begin Balancing with the Most Complex Molecule

In our example, let's start balancing with the molecule containing the most different elements - water (H2O):
- Since there are 2 hydrogen atoms on the right and 4 on the left, balance the hydrogens by placing a 2 in front of the H2O:
CH4 + O2 → CO2 + 2H2O
- Now, update the table with the new numbers:
| Element | Reactants | Products |
|---|---|---|
| Carbon (C) | 1 | 1 |
| Hydrogen (H) | 4 | 4 |
| Oxygen (O) | 2 | 4 |
💡 Note: Begin with the compound that has the least common denominators for the elements in it.
Step 4: Balance the Remaining Elements One at a Time

Next, balance the oxygen:
- Place a 2 in front of O2 to account for the 4 oxygen atoms in the products:
CH4 + 2O2 → CO2 + 2H2O
Now, the equation is:
| Element | Reactants | Products |
|---|---|---|
| Carbon (C) | 1 | 1 |
| Hydrogen (H) | 4 | 4 |
| Oxygen (O) | 4 | 4 |
🔍 Note: Use whole numbers for coefficients to keep calculations simpler.
Step 5: Double-Check Your Work
The balanced equation should now be:
CH4 + 2O2 → CO2 + 2H2O
Re-count the atoms to confirm:
| Element | Reactants | Products |
|---|---|---|
| Carbon (C) | 1 | 1 |
| Hydrogen (H) | 4 | 4 |
| Oxygen (O) | 4 | 4 |
All elements are now balanced!
In conclusion, mastering these five steps provides a solid approach to balancing chemical equations. With practice, the process becomes intuitive, allowing you to quickly grasp the stoichiometry of reactions. By following these steps, not only will you understand the principles of chemical reactions better, but you'll also develop a problem-solving mindset applicable in various scientific and real-world scenarios.
Can you balance an equation with fractions?

+
Yes, fractions can be used temporarily during balancing, but they should be converted to whole numbers to simplify the equation for practical purposes.
Why is balancing chemical equations important?

+
Balancing ensures the law of conservation of mass is upheld, showing that the number of atoms involved in a reaction remains constant.
Is there a shortcut to balancing equations?

+
There are several methods like the algebraic method, but the step-by-step approach is the most straightforward and universally applicable.



