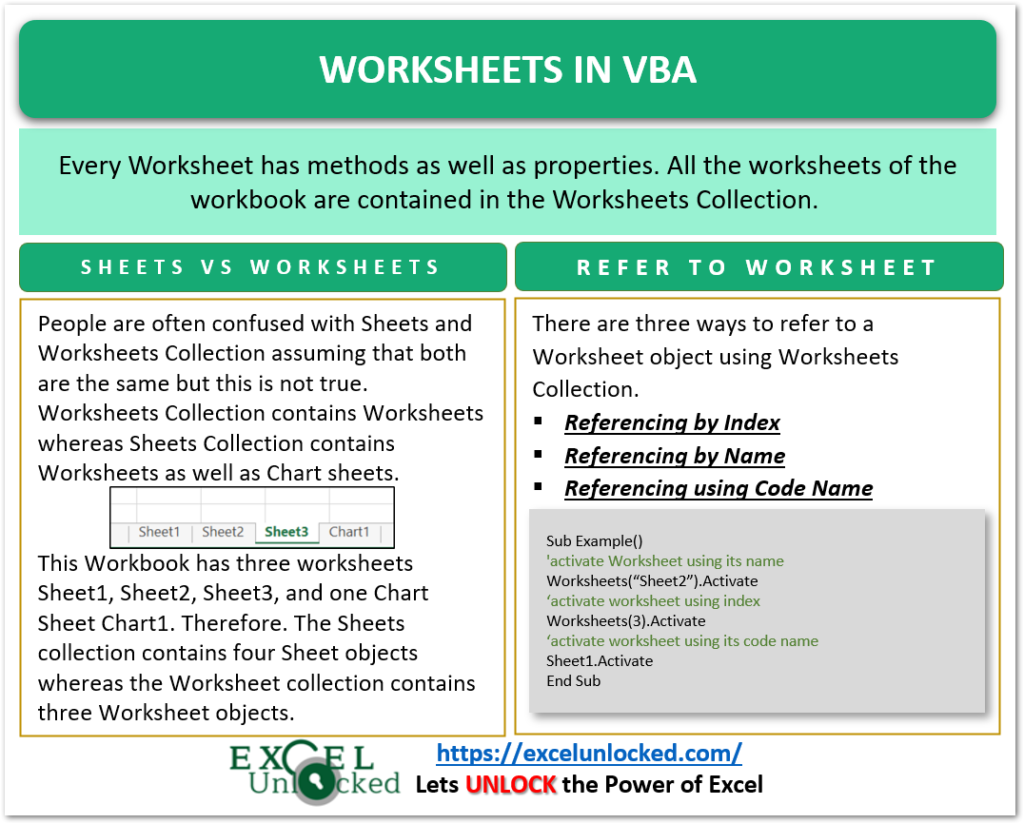
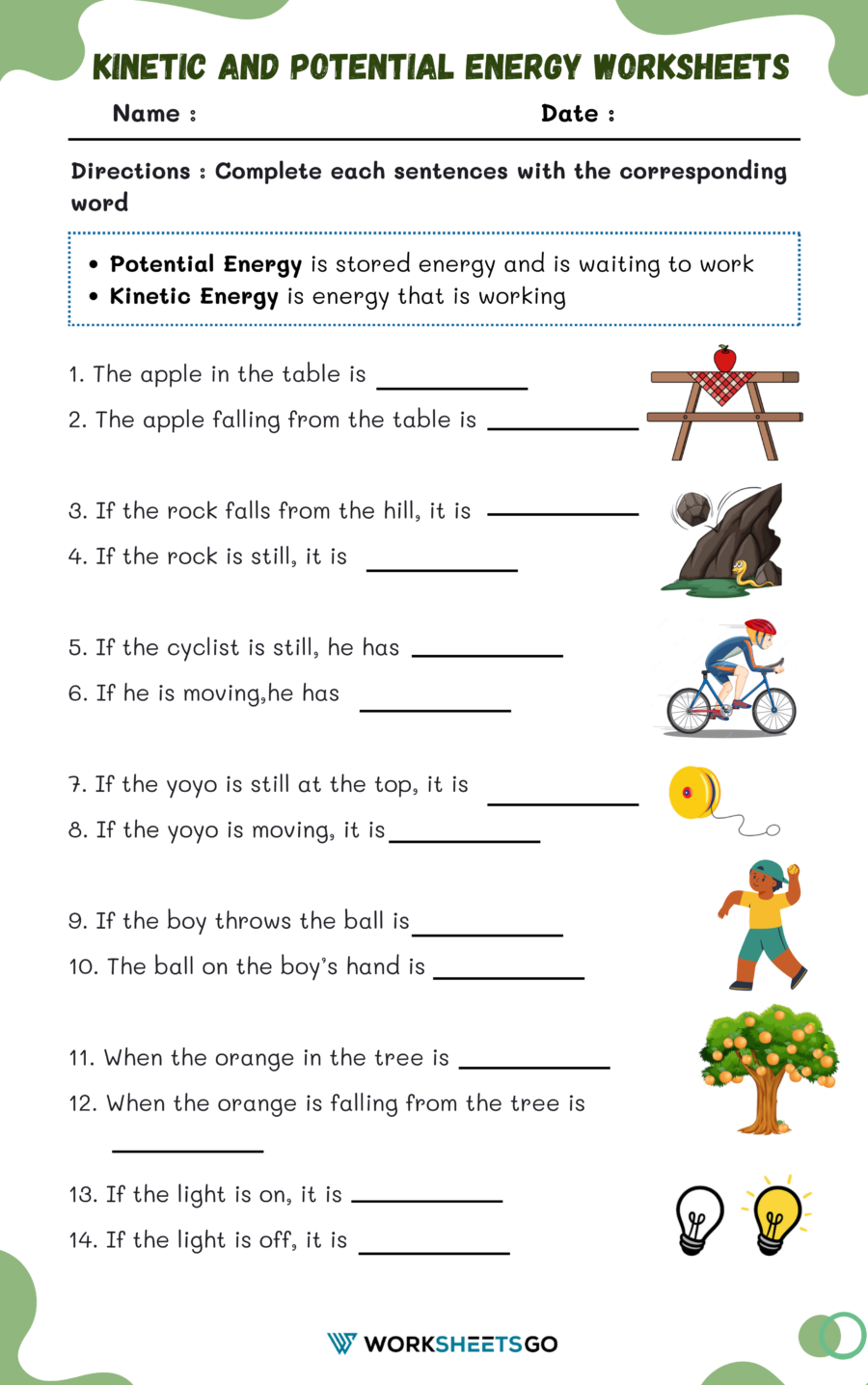
5 Essential Tips for Mastering Chemical Formulas
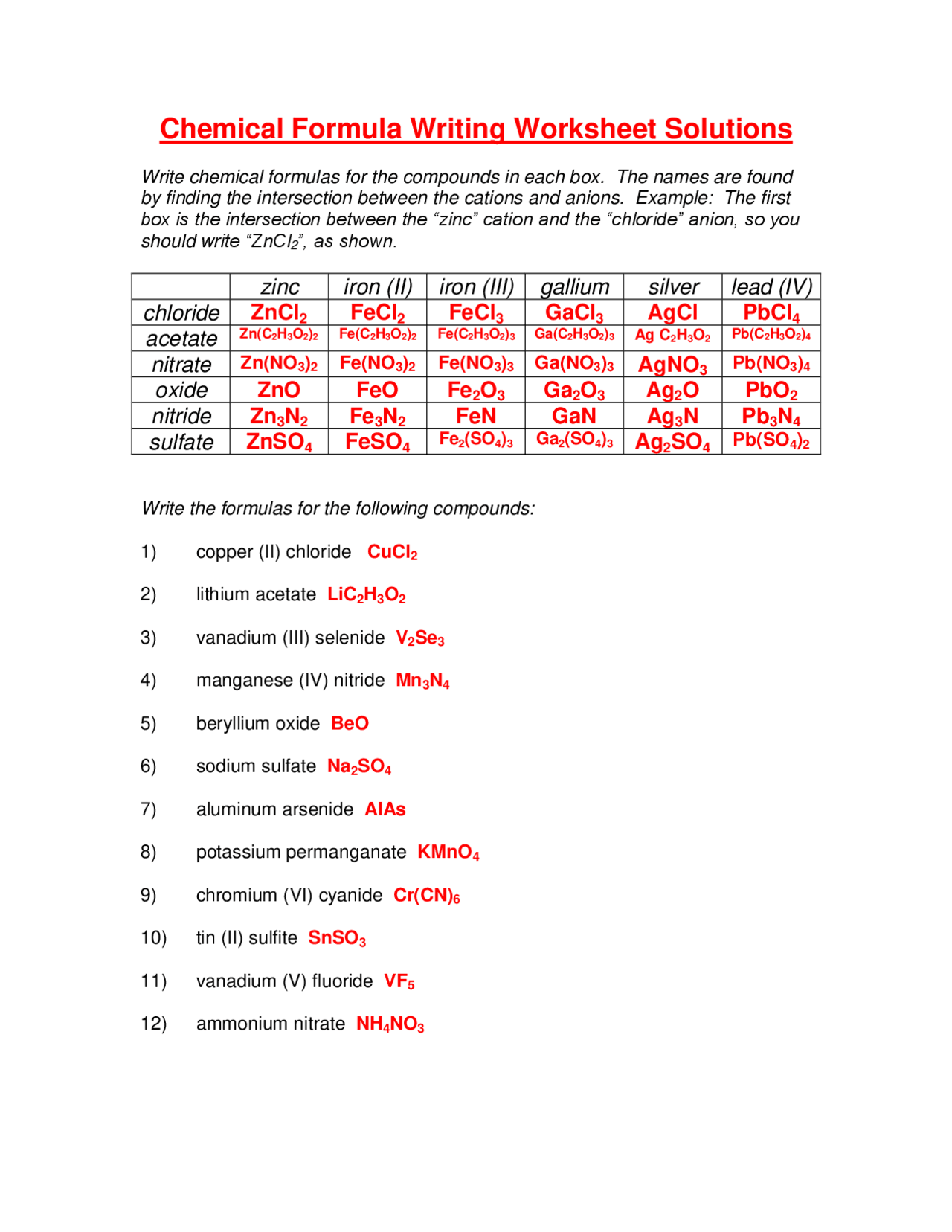
Understanding chemical formulas is essential for anyone delving into the world of chemistry, be it students, professionals, or enthusiasts. Chemical formulas serve as the shorthand for describing the composition of compounds, telling us the types of atoms in a molecule and the ratios in which they combine. Here are five essential tips that can help you master the art of interpreting and working with chemical formulas effectively.
1. Learn the Basics of Chemical Symbols
Before diving into the complex structures of chemical compounds, familiarize yourself with the periodic table. Each element has a unique symbol derived from its name or its Latin origin:
- H for hydrogen
- O for oxygen
- Fe for iron (Ferrum in Latin)
Knowing these symbols helps in understanding chemical formulas at their most fundamental level. Here’s a simple example to get you started:
🔬 Note: The symbol for sodium is Na (Natrium in Latin), not S, which might be confused with sulfur.
2. Understand the Concept of Molar Ratios

Chemical formulas provide insight into how many atoms of each element are present in a molecule. The ratio of atoms is crucial for understanding:
- Reaction stoichiometry
- Molecular mass calculations
- Balancing chemical equations
For instance, in the formula H2O, the 2 indicates that there are two hydrogen atoms for every one oxygen atom.
🔍 Note: Always consider the coefficients in front of compounds when balancing equations to maintain molar ratios.
3. Utilize Molecular Models for Visualization

One of the most effective ways to understand chemical formulas is to visualize the molecular structure:
- Use ball-and-stick models
- Employ software or apps that simulate molecular structures
- Construct physical models using kits
Seeing the spatial arrangement of atoms can significantly enhance your grasp on how compounds interact.
4. Practice Writing and Balancing Equations

The ability to write and balance chemical equations is a direct application of understanding chemical formulas:
- Start with simple reactions like combustion or formation reactions.
- Move to more complex processes like precipitation or acid-base reactions.
Here’s an example:
| Reactants | Products |
|---|---|
| CH4 + 2O2 | CO2 + 2H2O |

5. Integrate with Real-World Applications

To make your learning more engaging, relate chemical formulas to real-world applications:
- Explore pharmaceuticals: How do medications like aspirin work?
- Look into environmental chemistry: Understand the impact of pollutants like CO2 or NO2.
- Consider industrial uses: Discover how materials like steel or polymers are synthesized.
By connecting abstract concepts to tangible examples, chemical formulas come alive, becoming more memorable and understandable.
The mastery of chemical formulas opens up a universe of chemical phenomena, making it a fundamental skill in the science of chemistry. It not only allows you to predict the behavior of substances but also to innovate and solve practical problems. Remember, like any language, the language of chemistry becomes more intuitive and expressive with practice and application.
Why are chemical formulas important?

+
Chemical formulas are essential because they provide a concise way to communicate the composition of compounds, predict reactions, and understand chemical interactions.
How do I remember chemical symbols?

+
Associating each element’s symbol with its name, origin, or through mnemonic devices can help in memorizing them effectively.
What is the difference between empirical and molecular formulas?

+
An empirical formula shows the simplest whole-number ratio of atoms in a compound, while a molecular formula gives the actual number of atoms of each element in a molecule.
Can I use chemical formulas in daily life?

+
Absolutely! Understanding chemical formulas can help you comprehend product labels, cooking reactions, and even environmental issues like pollution and climate change.