5 Simple Steps to Balance Chemical Equations Easily

In the world of chemistry, balancing chemical equations is a fundamental skill that all students and chemists must master. A balanced equation ensures that the laws of conservation of mass and energy are not violated. This post will guide you through the process of balancing chemical equations in a simple, straightforward manner, making a complex concept accessible to everyone.
Understanding Chemical Equations


Before diving into the balancing process, let’s briefly understand what a chemical equation represents:
- Reactants: Substances that react with one another.
- Products: New substances formed as a result of the reaction.
- The equation itself depicts the reactants on the left, separated by an arrow pointing towards the products on the right.
Here’s an example:
| Reactants | → | Products |
| H2 + O2 | → | H2O |

Step 1: Identify the Reactants and Products

The first step in balancing any chemical equation is to identify all the reactants and products involved in the reaction. Here’s how you can do it:
- Write down the formula of each reactant and product.
- Count the number of atoms for each element on both sides of the equation.
🔍 Note: Always ensure to include all the atoms, including those in polyatomic ions or compounds, to avoid missing any components.
Step 2: Count the Atoms on Each Side

Now, let’s count the atoms:
- Hydrogen (H): 2 atoms on the left, 2 atoms on the right.
- Oxygen (O): 2 atoms on the left, 1 atom on the right.
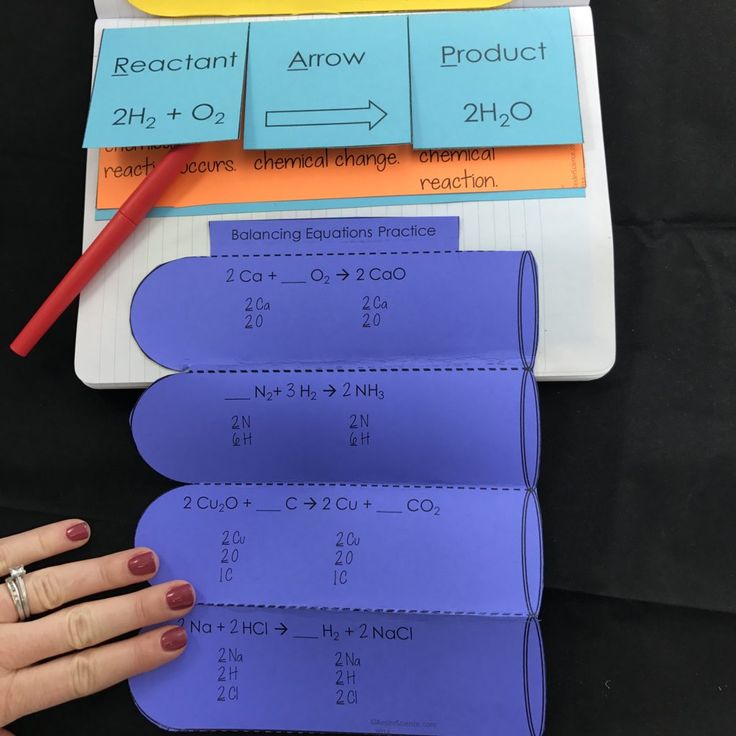
Step 3: Balance the Atoms

The goal is to have the same number of each type of atom on both sides:
- Start by balancing elements that appear in only one reactant and one product.
- Use coefficients to multiply the number of atoms for each compound.
For our example:
H2 + O2 → 2H2O
The equation is now balanced; we have 4 hydrogen atoms and 2 oxygen atoms on both sides.
Step 4: Check for Polyatomic Ions

In some reactions, polyatomic ions remain intact through the reaction. Here’s how to handle them:
- Identify any polyatomic ions that do not change during the reaction.
- Balance these ions as single units rather than individual atoms.
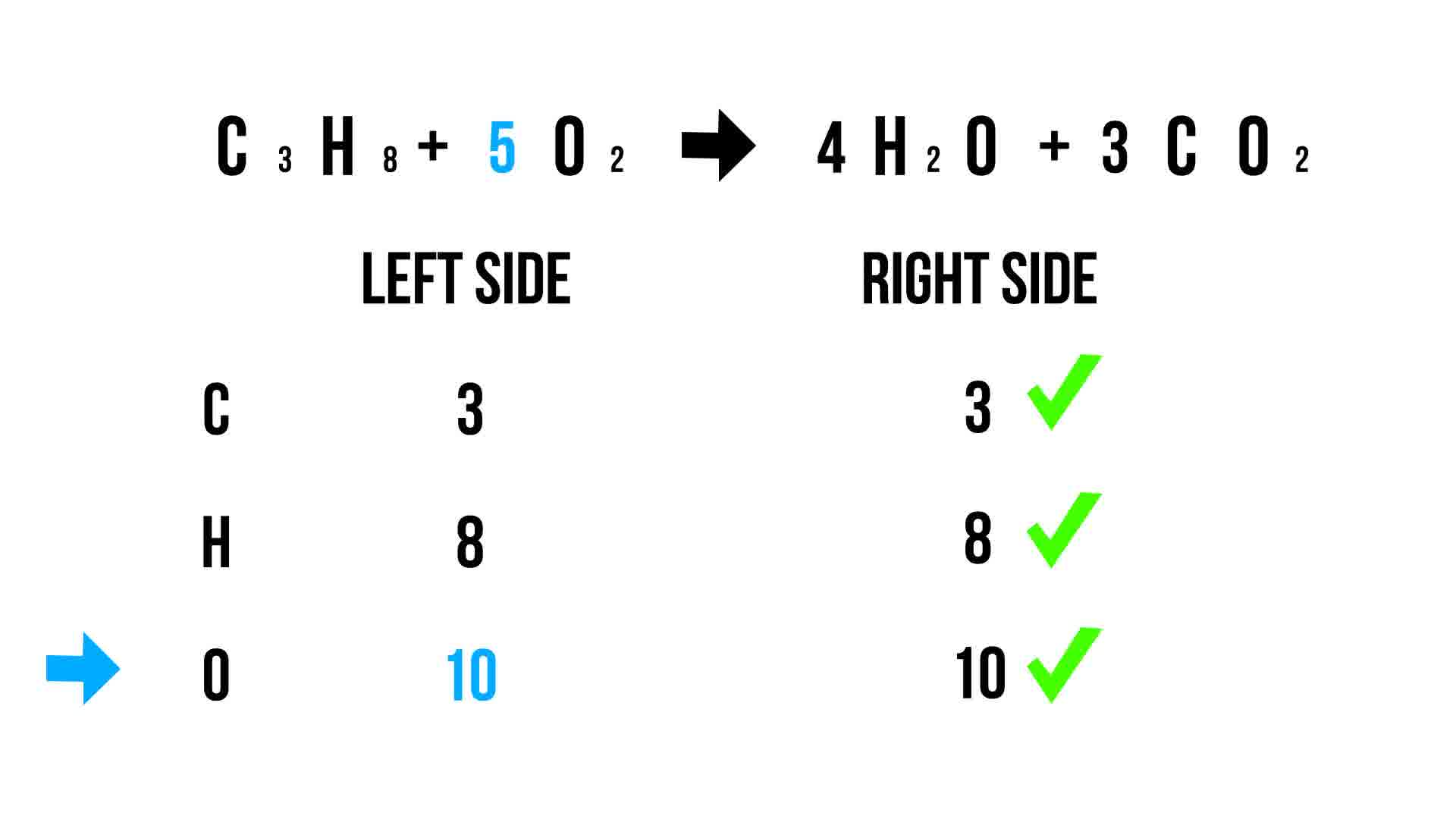
Step 5: Verify the Balance
Lastly, double-check to ensure that:
- All atoms are balanced.
- The equation obeys the law of conservation of mass.
✅ Note: It's beneficial to start with the most complex molecule and then work on the simpler ones to make balancing easier.
By following these steps, anyone can balance chemical equations efficiently. Understanding the principles behind balancing equations allows you to not only excel in chemistry classes but also comprehend the underlying structure of matter and reactions at a molecular level.
Why do we balance chemical equations?

+
Balancing chemical equations is essential to ensure that the law of conservation of mass is observed, meaning that matter is neither created nor destroyed in a chemical reaction.
What if I have fractions after balancing the equation?

+
If you end up with fractions, you can multiply every coefficient by the same number to get rid of the fraction, making the equation balanced with whole numbers.
Is it okay to change the subscripts of compounds to balance the equation?

+
No, changing the subscripts would mean altering the compound’s identity, which isn’t allowed in balancing reactions. Use only coefficients to balance atoms.
Can I balance an equation by balancing oxygen first?
+
Yes, you can start by balancing any element, but it’s often easier to leave oxygen and hydrogen for the end as they tend to appear more frequently in compounds.