Master Ionic Compounds: Easy Naming and Writing Guide
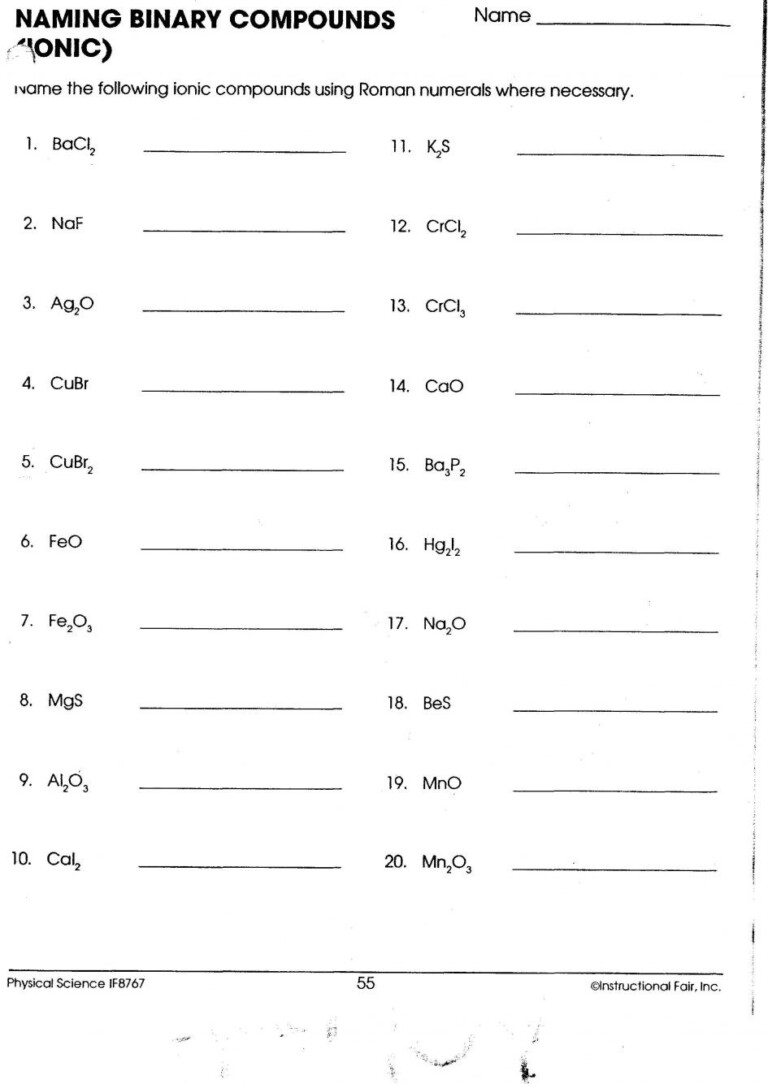
Ionic compounds are a fundamental part of chemistry, with their unique bonding mechanisms and properties playing crucial roles in both natural and industrial processes. Understanding how to name these compounds and write their formulas is essential for students, educators, and professionals alike. This guide will delve into the specifics of ionic compound naming, formula writing, and related concepts, providing a comprehensive look into the world of ionic chemistry.
Understanding Ionic Bonds
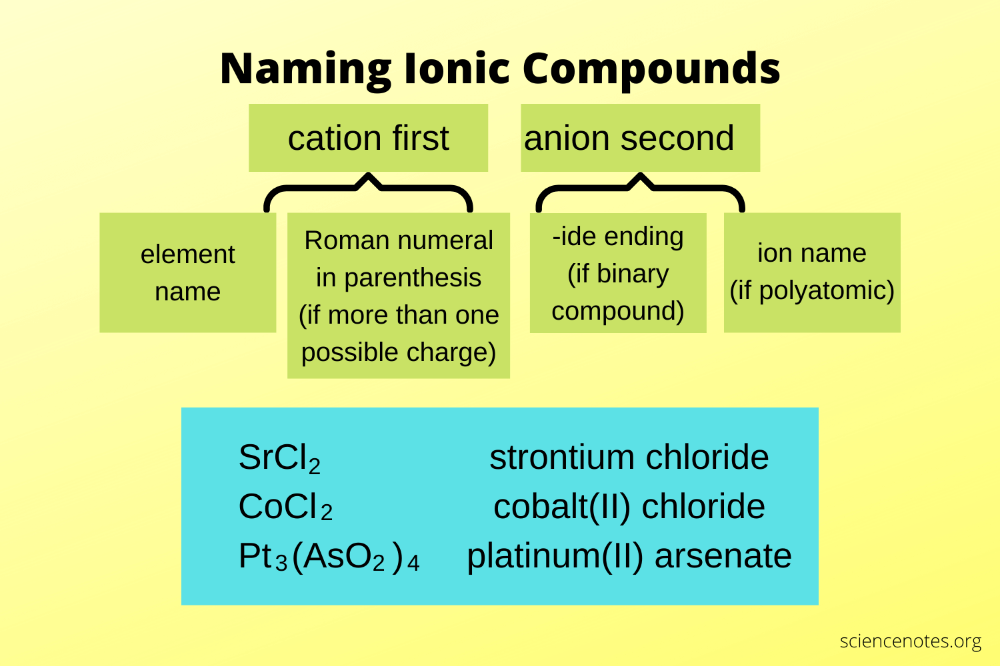
Ionic bonds occur between metals and nonmetals, where electrons are transferred from the metal to the nonmetal, creating oppositely charged ions. Here's how you can differentiate between ionic and covalent bonds:
- Metal + Nonmetal: Ionic bond.
- Nonmetal + Nonmetal: Covalent bond.
💡 Note: The rule isn't absolute but serves as a guideline. Some elements can exhibit different bonding behaviors under certain conditions.
Naming Ionic Compounds

Naming ionic compounds follows straightforward rules, with some exceptions for polyatomic ions:
Binary Ionic Compounds

For compounds consisting of two elements:
- Name the metal first, using its elemental name.
- Name the nonmetal last, but change its ending to '-ide'.
Example: NaCl is sodium chloride.
Compounds with Transition Metals

Transition metals can form multiple ions with different charges:
- Include the charge as a Roman numeral in parentheses if the metal has multiple common ions.
- Follow the same rule for the nonmetal anion.
Example: CuO could be copper(II) oxide or copper(I) oxide, depending on the charge of the copper ion.
Polyatomic Ions

Ions like NO3- (nitrate) or SO42- (sulfate) are treated as single units when naming:
- Name the metal as usual.
- Name the polyatomic ion as it is, without changing the ending.
Example: NaNO3 is sodium nitrate.
Here's a handy table for common polyatomic ions:
| Ion | Name | Formula |
|---|---|---|
| Nitrate | Nitrate | NO3- |
| Sulfate | Sulfate | SO42- |
| Carbonate | Carbonate | CO32- |

Writing Ionic Formulas

Writing the formula for an ionic compound involves balancing the charges to ensure the overall compound is neutral:
- Identify the ions and their charges.
- Write the metal first, followed by the nonmetal or polyatomic ion.
- Balance the charges by adjusting the number of ions until the total charge is zero.
Example: For calcium chloride, the charges are Ca2+ and Cl-, leading to CaCl2.
Naming and Formula Writing Tips
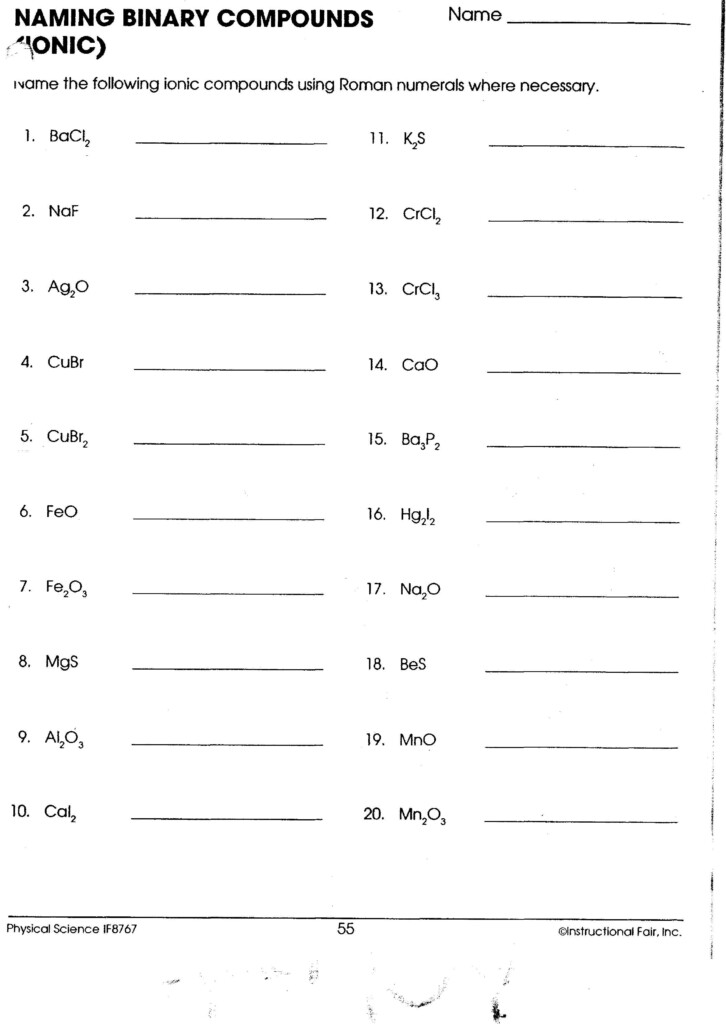
Here are some tips to keep in mind:
- Always check the charge of the ions to ensure the compound is electrically neutral.
- Use Roman numerals with transition metals when they have variable charges.
- Polyatomic ions retain their names; you do not change their endings.
🔔 Note: When dealing with polyatomic ions, remember that the formula might include parentheses if there are multiple polyatomic ions.
In summary, mastering ionic compounds involves understanding the rules for their formation, naming, and formula writing. Ionic compounds are pivotal in numerous applications, from medicine to technology, emphasizing the importance of this knowledge. Whether you're a student looking to excel in chemistry or a professional seeking to refresh your understanding, this guide provides the tools to confidently navigate ionic chemistry.
What are the key differences between ionic and covalent compounds?

+
Ionic compounds involve the transfer of electrons from metals to nonmetals, forming ions with opposite charges that attract each other, leading to high melting points and electrical conductivity when dissolved. Covalent compounds, in contrast, share electrons between nonmetals, often leading to lower melting points and molecular structures without conductivity in their pure form.
Can ionic compounds be gases or liquids at room temperature?

+
Generally, ionic compounds are solids at room temperature due to the strong ionic bonds. However, under certain conditions, some ionic compounds can form hydrates or exist in aqueous solutions, where they might appear as liquids. Ionic compounds like ammonia salts can also have lower melting points but are still primarily solid at room temperature.
What happens if the charges don’t balance when writing an ionic formula?

+
If the charges do not balance, the formula is incorrect. You need to adjust the number of each ion until the total positive charge equals the total negative charge. For example, if you have Al3+ and O2-, you need two aluminum ions for every three oxygen ions, resulting in Al2O3.