5 Tips for Solving Atomic Structure Worksheets

Understanding atomic structure is a fundamental concept in chemistry, essential for anyone studying the subject. Whether you're a student grappling with homework or an enthusiast looking to better comprehend the world of atoms, mastering worksheets on atomic structure can be incredibly beneficial. Here are five tips to help you excel in solving atomic structure worksheets:
1. Understand the Basics of Atomic Structure

Before diving into worksheets, ensure you have a solid grasp of the basic components of an atom:
- Nucleus: Contains protons and neutrons. Protons are positively charged, while neutrons have no charge.
- Electrons: Negatively charged particles that move around the nucleus in shells or orbitals.
- Atomic Number: Represents the number of protons in the nucleus, which also defines the element's identity.
- Mass Number: Sum of protons and neutrons in an atom. Isotopes of an element have the same atomic number but different mass numbers due to varying neutron counts.
📚 Note: An element's atomic number does not change; it's the neutron count that varies among isotopes.
2. Master the Periodic Table
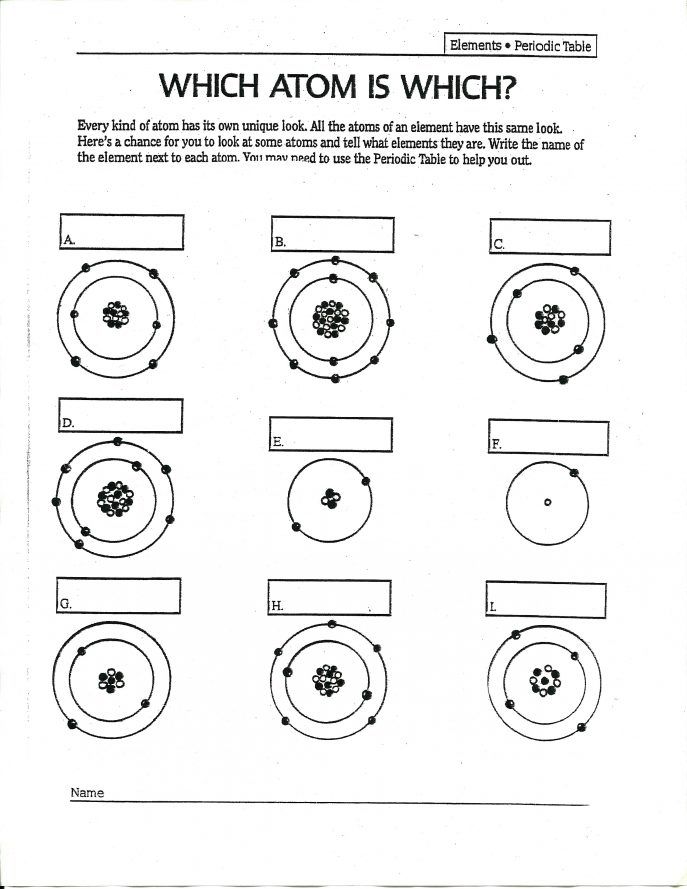
The periodic table is your best friend when dealing with atomic structure:
- Rows (periods): Indicate the number of shells where electrons can be found.
- Columns (groups): Denote the number of valence electrons, which are crucial for understanding an element's chemical behavior.
- Atomic Symbols: Represent elements with a letter or two, often with the atomic number above and the mass number below.
- Atomic Mass: Listed under each element, it's an average mass considering all isotopes and their abundance.
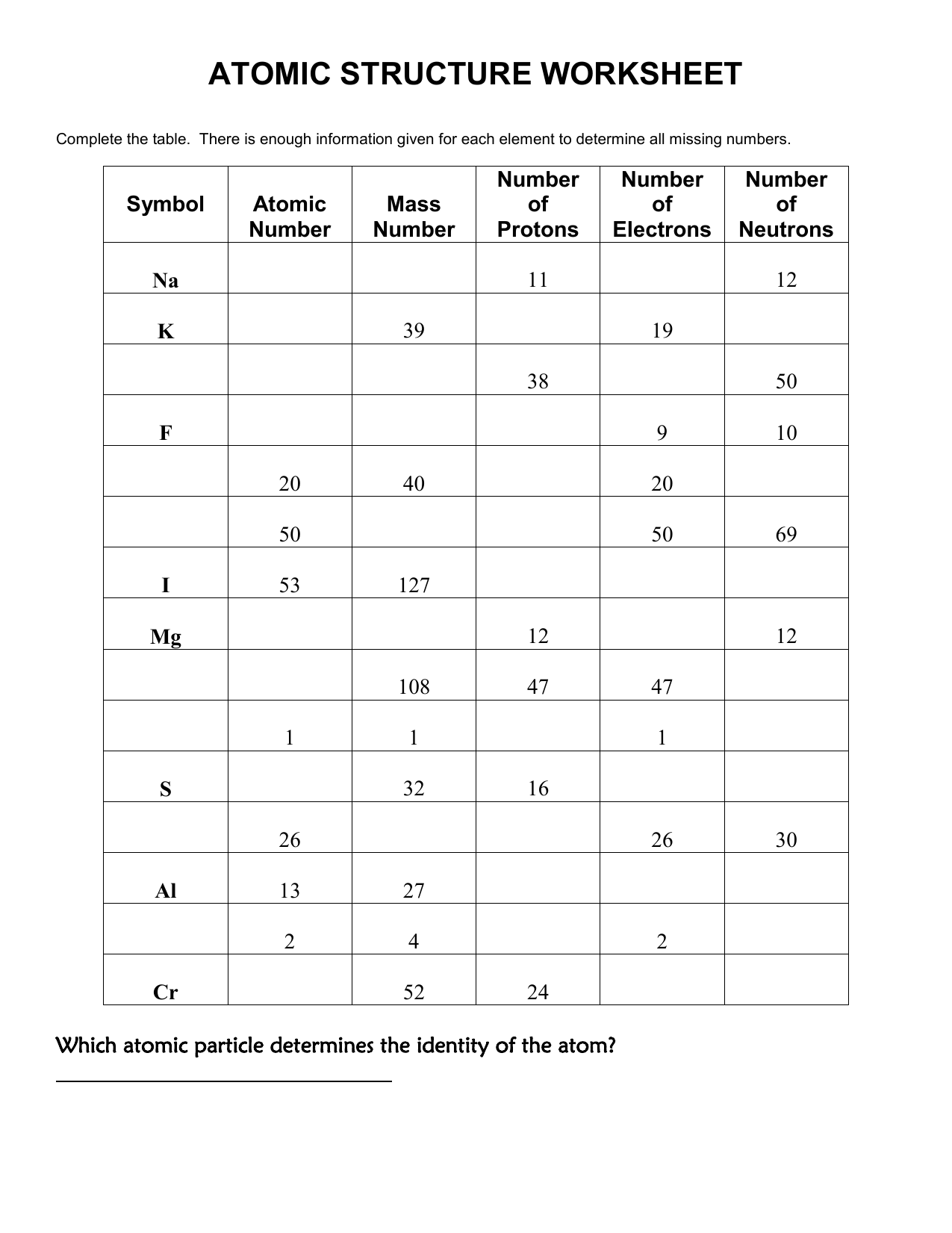
| Element | Atomic Number | Atomic Symbol | Mass Number |
|---|---|---|---|
| Hydrogen | 1 | H | 1 |
| Helium | 2 | He | 4 |
| Lithium | 3 | Li | 7 |

3. Practice Electron Configuration
Electron configuration describes how electrons are distributed among the various atomic orbitals:
- Use the Aufbau principle, Hund's rule, and the Pauli exclusion principle to determine electron placement.
- Aufbau Principle: Electrons fill orbitals starting from the lowest energy level to the highest.
- Hund's Rule: Electrons occupy orbitals of the same energy so that each orbital acquires one electron before any orbital acquires a second.
- Pauli Exclusion Principle: No two electrons in an atom can have the same set of quantum numbers.
⚠️ Note: Electron configurations can be quite complex, so keep practicing the rules to understand how they apply to different elements.
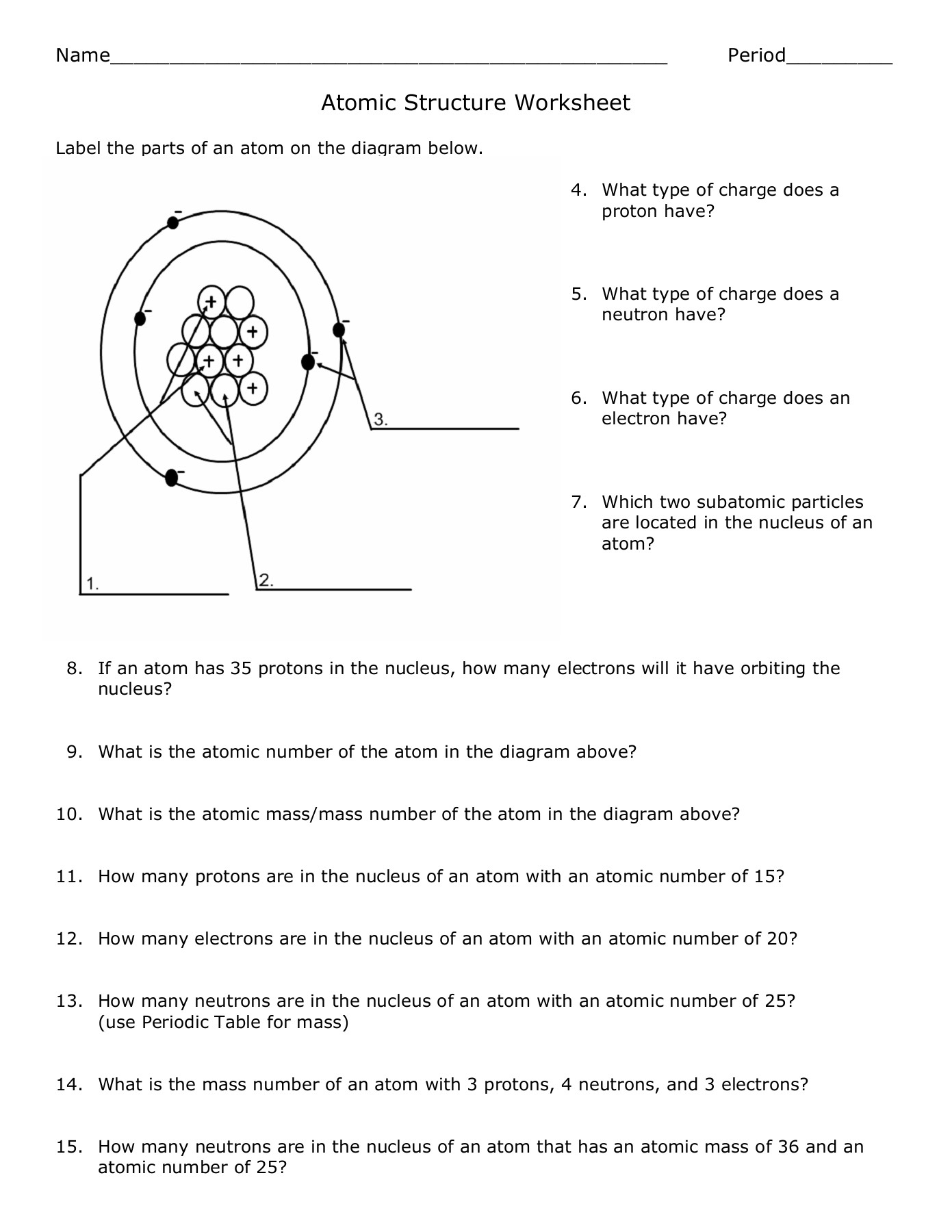
4. Leverage Diagrams and Models

Visual representations can significantly aid in understanding atomic structure:
- Bohr Model: Depicts electrons in orbits or shells around the nucleus.
- Lewis Dot Structures: Use dots to represent the valence electrons, which are critical for bonding and reactivity.
- Energy Level Diagrams: Show how electrons fill energy levels or subshells.
Here is an example of a Lewis Dot Structure for Nitrogen:
N:
• • • N • • •
5. Review and Practice

Consistent review and practice are key to mastery:
- Do not just solve problems; review the steps and understand why they work.
- Use a variety of resources like textbooks, online videos, or interactive apps to learn from different perspectives.
- Join study groups or forums where you can discuss questions and solutions with peers.
- Take practice tests under timed conditions to simulate exam settings.
🧠 Note: Make it a habit to review mistakes and misconceptions to strengthen your understanding.
As you incorporate these tips into your study routine, you'll find that atomic structure worksheets become less daunting. Remember, the goal is not just to solve problems but to understand the underlying principles of chemistry. By applying these strategies, you'll enhance your ability to grasp complex concepts, improve your grades, and lay a solid foundation for advanced topics in chemistry.
What is the importance of understanding atomic structure in chemistry?

+
Understanding atomic structure is crucial as it forms the basis for explaining how elements interact, bond, and form compounds. It explains chemical reactions, reactivity, and the periodic trends of elements.
Can I skip learning about electron configuration?
+
No, learning electron configuration is essential as it provides insights into an element’s chemical properties and how it will bond with other elements. It’s fundamental for understanding chemical periodicity and reactivity.
How often should I practice atomic structure worksheets?
+
Regular practice is key. Aim for at least 2-3 times a week if you’re new to the concept. As you become more proficient, keep practicing to maintain and sharpen your skills.



