Equilibrium Constants Chem Worksheet 18-3 Answer Key Revealed

Understanding the Basics of Equilibrium Constants

Chemical equilibrium is a fundamental concept in chemistry, involving the state where the concentrations of reactants and products remain constant over time. This balance is characterized by the equilibrium constant, which provides insight into the extent of a chemical reaction at equilibrium. Here’s what you need to know:
- Definition: The equilibrium constant (K) is a measure of the ratio of product concentrations to reactant concentrations at equilibrium.
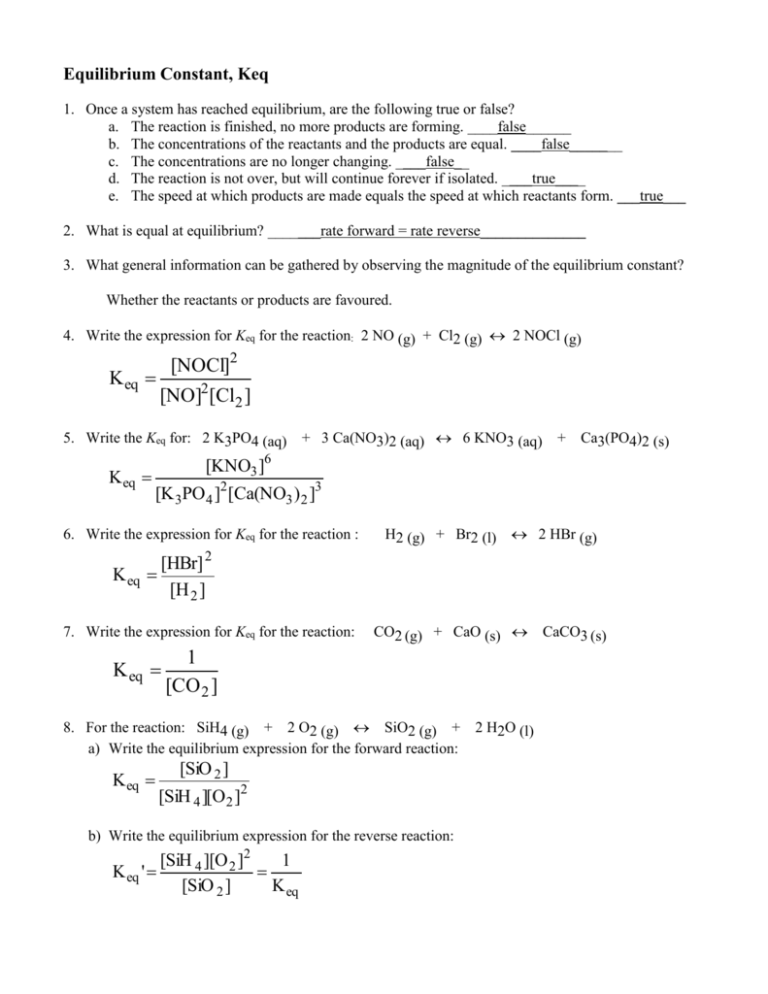
- Mathematical Expression: For a generalized reaction where aA + bB ⇌ cC + dD, the equilibrium constant expression is K = [C]^c * [D]^d / [A]^a * [B]^b, where [A], [B], [C], and [D] are molar concentrations at equilibrium.
📝 Note: The equilibrium constant does not include solids or pure liquids in its expression since their activities are unity (1).
Equilibrium Constant Calculations

Calculating equilibrium constants involves a series of steps:
- Write the Equilibrium Expression: Identify the stoichiometric coefficients and set up the equilibrium constant expression.
- Determine Initial and Equilibrium Concentrations: Use given or calculated values to find the concentration of each component at equilibrium.
- Calculate K: Substitute the concentrations into the expression and solve for K.
Let's illustrate this with an example:
Consider the reaction: H2(g) + I2(g) ⇌ 2HI(g), with the following equilibrium concentrations: [H2] = 0.1 mol/L, [I2] = 0.1 mol/L, and [HI] = 0.8 mol/L.
| Substance | Concentration at Equilibrium (mol/L) |
|---|---|
| H2 | 0.1 |
| I2 | 0.1 |
| HI | 0.8 |

The equilibrium constant expression for this reaction is K = [HI]2 / ([H2] * [I2]):
Substituting the concentrations, we get:
K = (0.82) / (0.1 * 0.1) = 64
🌟 Note: The equilibrium constant is unitless since it's a ratio of concentrations.
Interpreting the Equilibrium Constant

The value of K provides essential information:
- K > 1: The reaction favors the formation of products.
- K < 1: The reaction favors the reactants.
- K ≈ 1: Reactants and products are present in nearly equal amounts.
Equilibrium Constant Worksheet 18-3: Explanation and Answer Key

Here's a step-by-step guide to solving problems from Worksheet 18-3:
Problem 1:

Given the reaction: N2(g) + 3H2(g) ⇌ 2NH3(g) with initial concentrations [N2] = 2.0 M, [H2] = 3.0 M, and at equilibrium [NH3] = 1.5 M, calculate K.
- Set up the expression: K = [NH3]2 / ([N2] * [H2]3).
- Determine the concentrations at equilibrium:
- [N2] = 2.0 M - x, where x is the concentration of N2 reacted to form NH3.
- [H2] = 3.0 M - 3x
- Since [NH3] = 1.5 M = 2x, x = 0.75 M
- Substitute the values into the expression:
K = (1.52) / ((2.0 - 0.75) * (3.0 - 3*0.75)3)
K ≈ (2.25) / (1.25 * 0.753) ≈ 5.33
Problem 2:

Given: PCl5(g) ⇌ PCl3(g) + Cl2(g) with [PCl5] = 0.5 M and [Cl2] = 0.2 M at equilibrium. Calculate [PCl3] if K = 0.33.
Step-by-Step:
- Set up the expression: K = [PCl3] * [Cl2] / [PCl5]
- Substitute known values into the expression:
0.33 = [PCl3] * 0.2 / 0.5
- Solve for [PCl3]:
[PCl3] = 0.33 * 0.5 / 0.2 = 0.825 M
Importance of the Equilibrium Constant in Chemistry
The equilibrium constant is not just a numerical value; it's a dynamic indicator of how chemical reactions proceed:
- Predicting Reaction Extent: It helps predict how far a reaction will proceed before equilibrium is reached.
- Effect of Temperature: Changes in K due to temperature variations inform about reaction's thermodynamic properties.
- Shifting Equilibrium**: Le Chatelier's principle uses changes in K to explain why reactions shift to accommodate changes in conditions.
🌡️ Note: Temperature changes affect K, but not necessarily in a straightforward manner. Exothermic reactions typically see a decrease in K with an increase in temperature.
Understanding equilibrium constants is vital for controlling reaction conditions, optimizing industrial processes, and predicting how chemical systems will respond to external changes. Whether you're synthesizing ammonia for fertilizers or studying protein folding, knowing the equilibrium constant gives you power over the molecular world.
What does a high equilibrium constant (K > 1) signify?

+
A high K value indicates that the reaction strongly favors the formation of products at equilibrium. The products will be in greater concentration compared to the reactants.
Can equilibrium constants change?

+
The equilibrium constant does change with temperature due to the alteration in the reaction’s enthalpy. Concentration changes, pressure changes for gases, or addition of catalysts do not change K but might shift the equilibrium position.
Why are solids and pure liquids excluded from K expressions?

+
Their concentrations (or more accurately, their activities) are considered constant and, therefore, are conventionally set to unity in equilibrium expressions.



