Unlock Chemistry Secrets: Bohr Models Worksheet Answers

Understanding chemistry isn't just about memorizing formulas or equations; it's about grasping the fundamental structure of atoms and how they interact. One way to achieve this understanding is through the Bohr model, named after Danish physicist Niels Bohr, who proposed an atomic theory that revolutionized our comprehension of atomic structure. In this blog post, we will delve into unlocking the chemistry secrets by exploring Bohr Models Worksheet answers, offering insights into how these models illustrate electron behavior and their significance in modern chemistry.
The Bohr Model of Atom
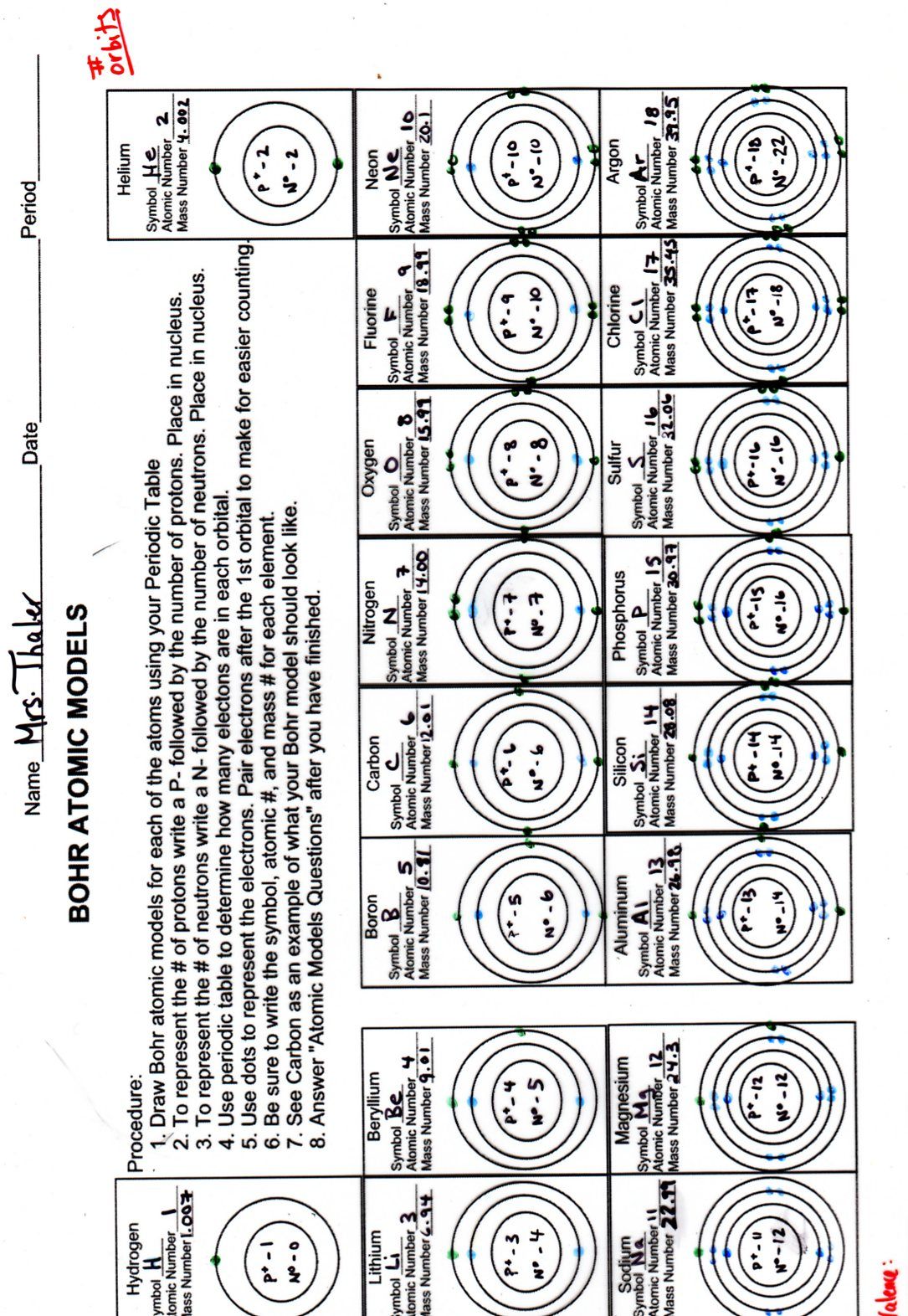
The Bohr model simplifies the complex reality of atomic structure by presenting electrons as orbiting the nucleus in defined energy levels or shells. Each shell corresponds to a specific energy level, and these levels are quantized, meaning that electrons can only exist at certain distances from the nucleus, not in between.
How the Bohr Model Works
- Energy Levels: Electrons are arranged in shells, often visualized as concentric circles, with the innermost shell being the first energy level (n=1), followed by the second level (n=2), and so on.
- Electron Capacity: Each shell can hold a maximum number of electrons calculated by the formula 2n^2, where n is the shell number. For example, the first shell can hold up to 2 electrons, the second up to 8, and the third up to 18.
- Transitions: Electrons can jump between shells, but they must absorb or emit energy in packets called quanta. These transitions are responsible for the light emission/absorption spectra observed in atomic spectroscopy.
Application in the Bohr Models Worksheet

The worksheet typically involves:
- Determining the number of electrons in each energy level for given atoms.
- Placing electrons in shells according to the capacity rule.
- Understanding the behavior of electrons when transitioning between energy levels.
Practical Steps to Solve Bohr Model Worksheets
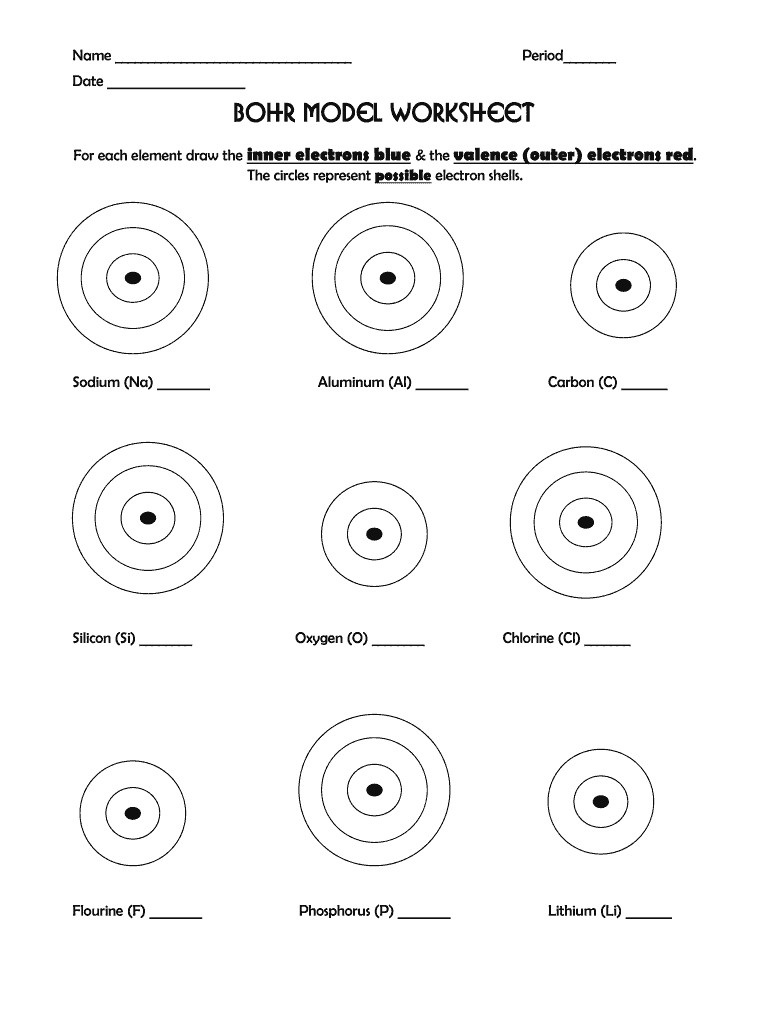
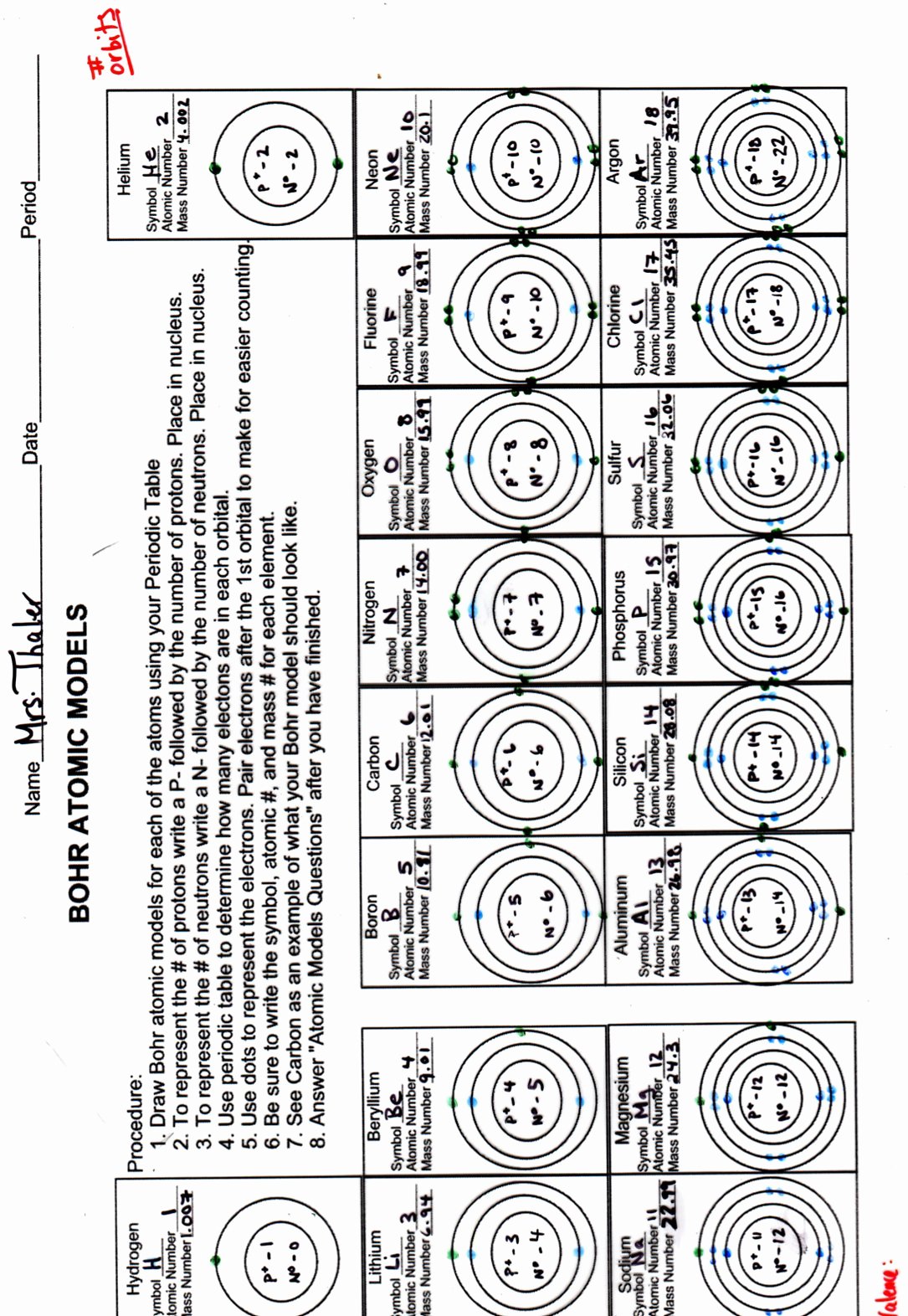
Step 1: Identify the Atomic Number
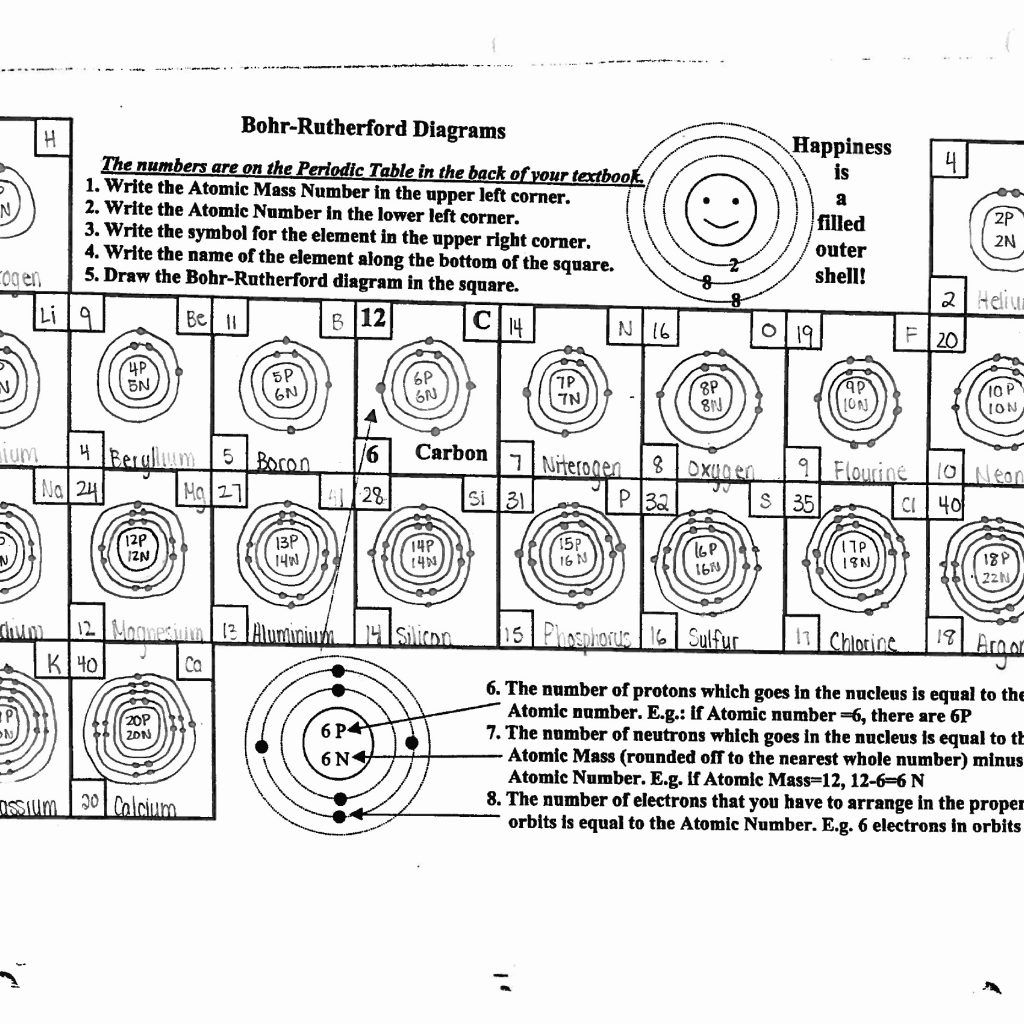
Every atom has a unique atomic number which determines its number of protons, and hence, its number of electrons in a neutral state. Here, you locate the element on the periodic table to find its atomic number.
Step 2: Constructing the Bohr Model

Here’s how to draw a Bohr model:
- Draw the nucleus. Represent protons and neutrons by writing "P" and "N" inside.
- Draw concentric circles to represent electron shells.
- Fill in the electrons:
- The first shell can hold up to 2 electrons (1s).
- The second shell can hold up to 8 electrons (2s and 2p).
- The third shell can hold up to 8 or 18 electrons depending on the element.
- Follow the rule of electron shell capacity: 2n^2.
- Place electrons in shells from innermost to outermost until you reach the number of electrons in the atom.
Step 3: Understanding Electron Transitions

Worksheets often ask about transitions, where electrons jump to different energy levels:
- If an electron absorbs energy, it moves to a higher energy level.
- When it drops back down, it releases energy as light, which can be observed in various spectra.
⚛️ Note: While the Bohr model simplifies the complex nature of quantum mechanics, it's crucial to understand that this model has limitations, especially when dealing with larger atoms or ions. It was refined by later models like the quantum mechanical model of the atom.
Examples and Solutions from Bohr Models Worksheet

Let's illustrate some common worksheet questions with answers:
Example 1: Helium (Atomic Number 2)

- Protons: 2
- Electrons: 2
- Setup: Two electrons in the first shell, which is full.
Example 2: Sodium (Atomic Number 11)
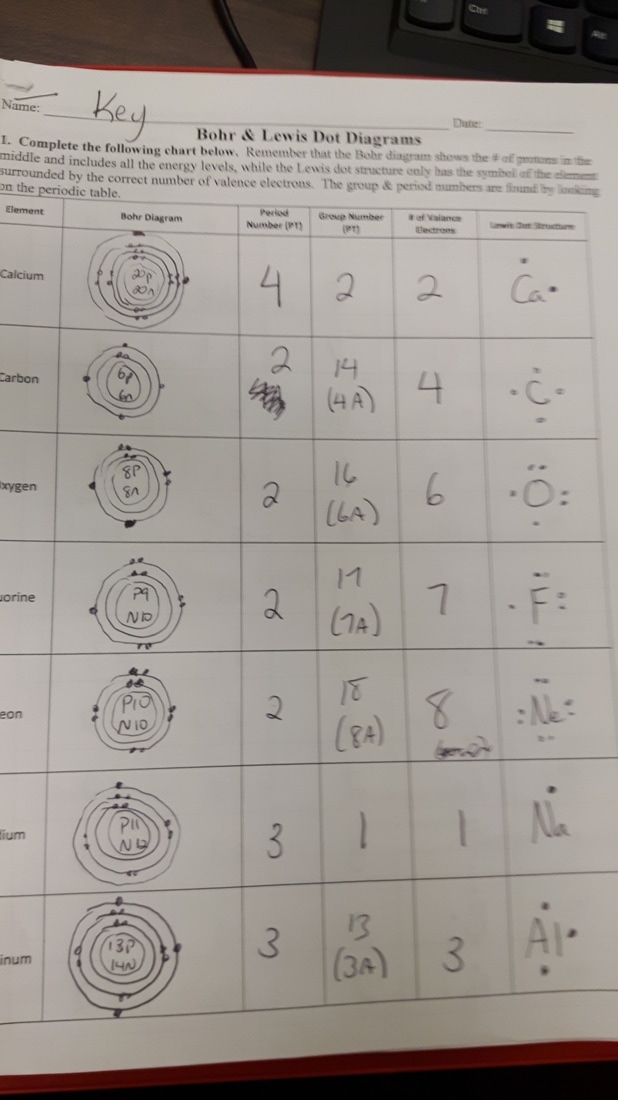
- Protons: 11
- Electrons: 11
- Setup:
- First Shell: 2 electrons
- Second Shell: 8 electrons
- Third Shell: 1 electron (this single electron in the third shell is responsible for sodium's chemical reactivity).
Importance of Bohr Models in Chemistry Education

The Bohr model, despite its simplifications:
- Helps visualize electron behavior, facilitating understanding of chemical bonding.
- Introduces students to quantum theory by linking the physical to the conceptual.
- Provides a foundation for understanding atomic structure before delving into more complex theories.
It's an essential tool that serves as a bridge between introductory chemistry concepts and advanced quantum mechanics, making it an invaluable teaching resource.
By working through Bohr Models Worksheets, students gain not just practical skills in drawing atomic structures, but also an intuitive understanding of how atoms work, which is crucial for further studies in physics, chemistry, and material science.
Summing up, Bohr Models Worksheet answers unlock various chemistry secrets, offering students a clearer picture of atomic structure and behavior. They empower students with the knowledge to understand why elements have certain chemical properties, how they might react, and how their electrons are arranged. This foundational knowledge not only aids in academic pursuits but also in understanding real-world phenomena where atomic interactions are at play. The simplicity of the Bohr model allows for a gentle introduction into the complex world of atomic theory, encouraging a deeper exploration into the fascinating world of chemistry. Remember, while this model has its limitations, it provides a stepping stone towards understanding the more comprehensive and accurate models like the quantum mechanical model, setting the stage for lifelong learning in the sciences.
Why is the Bohr model still used in chemistry education despite its limitations?
+
Despite its limitations, the Bohr model is still used because it provides a simple, visual introduction to atomic structure. It helps students understand basic concepts like electron shells, atomic energy levels, and the reasons behind atomic spectra before they encounter more complex quantum mechanical models.
How do Bohr models relate to an element’s chemical properties?

+
The placement of electrons in the outermost shell or valence shell of the Bohr model gives insight into an element’s reactivity. Elements with nearly full or nearly empty valence shells tend to be more reactive, as they seek to achieve a stable configuration, either by losing, gaining, or sharing electrons.
Can Bohr models predict the shape of molecules?

+
While Bohr models don’t directly predict molecular shapes, they give clues about bonding by indicating the number of valence electrons, which can influence bonding patterns. Advanced models like the VSEPR theory are used to predict molecular shapes more accurately.