5 Essential Tips for Nuclear Equation Balancing Answers
Understanding the Basics of Nuclear Equations
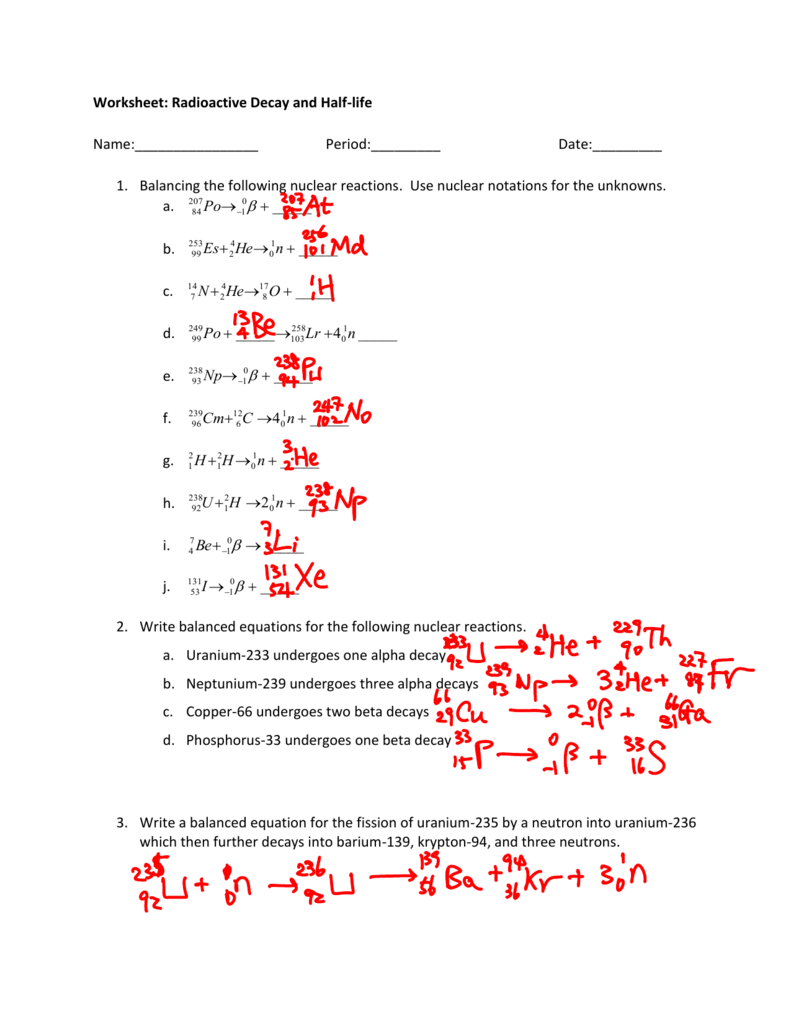
Nuclear reactions and equations involve particles that interact within an atomic nucleus. Understanding these processes is crucial for various scientific applications, from power generation to medical treatments like radiotherapy. Here’s how you can approach balancing nuclear equations with some essential tips.
Tip #1: Mass Number and Atomic Number Conservation

The first and perhaps the most fundamental step in balancing nuclear equations is understanding the conservation of the mass number (A) and atomic number (Z). Here’s what you need to do:
- Ensure the sum of the mass numbers on the reactants’ side equals the sum on the product side.
- Similarly, ensure the sum of the atomic numbers (or the total nuclear charge) is the same on both sides of the equation.
💡 Note: If you find any discrepancies in these totals, you've likely missed a particle, like a neutron or an electron.
Tip #2: Recognizing Common Nuclear Particles

Every nuclear reaction involves particles. Here are some common ones:
- Protons: (p, H-1)
- Neutrons: (n, 0n1)
- Alpha Particles: (α, He-4)
- Beta Particles: (β, e- for β- decay, e+ for β+ decay)
- Gamma Rays: (γ, no mass or charge)
Familiarize yourself with these, as they'll help in identifying missing particles when balancing the equation.
Tip #3: Using Algebra to Balance Equations

Sometimes, you might need to use algebra to find unknown elements or particles in nuclear equations. Here’s how:
- Assign variables like X or x to unknown elements/particles.
- Set up equations based on the conservation of mass number and atomic number.
- Using these equations, solve for the unknown components.
| Equation | Conservation Principle | Example |
|---|---|---|
| A_X → B + C | A = B_mass + C_mass | 238_X → 4_He + Y → X_mass = 238, He_mass = 4; Y_mass = 234 |
| A_X → B + C | Z_X = Z_B + Z_C | 238_U_92 → 4_He_2 + Y → U_charge = 92, He_charge = 2; Y_charge = 90 |

🔍 Note: When you use algebra, remember that an unknown particle's mass and charge must also obey the conservation rules.
Tip #4: Using Conservation of Energy and Momentum

While the focus is often on mass and atomic number conservation, considering energy and momentum conservation can also provide insight into the nature of nuclear reactions. Here are some considerations:
- Energy released (Q-value) should be considered when balancing nuclear reactions. Exothermic reactions release energy, while endothermic ones require input energy.
- Momentum before and after the reaction should also balance.
Tip #5: Applying Nuclear Chart of Nuclides

The chart of nuclides is an incredibly useful resource for any nuclear physicist or chemist. Here’s how it helps:
- It provides a graphical representation of isotopes and their stability, showing decay pathways.
- From the chart, you can deduce possible reaction outcomes or missing particles by tracing decay paths.
📊 Note: The chart of nuclides is often available in academic libraries or online nuclear physics resources.
Summary of Key Points

In this guide, we’ve covered five essential tips to balance nuclear equations. The primary focus has been on the conservation of mass number and atomic number, recognizing particles involved in nuclear reactions, using algebra to balance equations, considering energy and momentum, and leveraging the chart of nuclides. Each tip provides a unique approach to ensure the balance of a nuclear equation, keeping in mind the fundamental laws governing nuclear physics.
Why is the conservation of mass and atomic numbers important in nuclear equations?

+
This conservation ensures that the total amount of matter and charge remains constant in a nuclear reaction, reflecting the fundamental principles of physics.
Can particles appear or disappear in nuclear reactions?

+
No, while particles might transform or combine, the overall mass number and atomic number must be conserved, ensuring no particle is created or destroyed, just rearranged or altered.
How do I know which particles are involved in a nuclear reaction?

+
By identifying the reactants and products, and using the chart of nuclides or prior knowledge of common nuclear particles and reactions, you can determine which particles are likely involved.
What if my equation doesn’t balance?

+
First, check if you’ve accounted for all particles, including neutrons and other fundamental particles. Use algebra or the chart of nuclides to find missing particles or correct errors.
What role does energy conservation play in nuclear reactions?

+
Energy conservation helps determine if a reaction is exothermic or endothermic and provides insight into how energy is redistributed or released during the nuclear processes.