Balancing Equations: Free Chemistry Practice Worksheet

In the realm of chemistry, balancing chemical equations is a fundamental skill that every student must master. This process not only ensures that the law of conservation of mass is upheld but also helps in understanding the reaction mechanisms, stoichiometry, and yield calculations. Here, we'll explore a comprehensive guide to mastering the art of balancing chemical equations, complete with practical tips, steps, and a free practice worksheet to test your skills.
Understanding Chemical Equations

Before we dive into balancing, let’s clarify what a chemical equation represents:
- Reactants are the substances that react or interact to form new compounds.
- Products are the substances that are formed as a result of the chemical reaction.
A chemical equation is a symbolic representation of a chemical reaction. Each molecule involved in the reaction is written with its chemical formula, and arrows indicate the direction in which the reaction proceeds. For example:
CH4 + O2 → CO2 + H2O
The Importance of Balancing Equations

Balancing chemical equations is not just a box to tick; it has significant scientific implications:
- Conservation of Mass: Ensuring all atoms of each element on both sides of the equation are equal.
- Quantitative Information: Balanced equations allow for calculations like the mass of reactants needed or the volume of gases involved.
- Reaction Stoichiometry: Understanding the ratio in which reactants combine and products form.
Steps to Balance Chemical Equations

Here is a methodical approach to balancing chemical equations:
1. Write Down the Unbalanced Equation

Start by writing the skeleton equation where the reactants and products are listed with their chemical formulas, but not yet balanced. Example:
Fe + Cl2 → FeCl3
2. Count the Atoms

Count the number of atoms of each element on both sides of the equation:
| Element | Reactant Side | Product Side |
|---|---|---|
| Iron (Fe) | 1 | 1 |
| Chlorine (Cl) | 2 | 3 |

3. Use Coefficients to Balance

Adjust the coefficients (small whole numbers) in front of the molecules to make the number of atoms of each element equal on both sides. Keep in mind:
- Never change subscripts because that would alter the identity of the compound.
- Start with elements that appear in compounds on one side and as a free element on the other, like Cl in the example.
The balanced equation looks like:
2Fe + 3Cl2 → 2FeCl3
4. Check for Least Common Multiples

When possible, use the least common multiple to keep the coefficients as simple as possible. This step often involves trial and error.
5. Verify

Double-check that the numbers of all atoms on both sides of the equation are the same.
💡 Note: Balancing might require patience, especially with complex reactions. Always recheck your work.
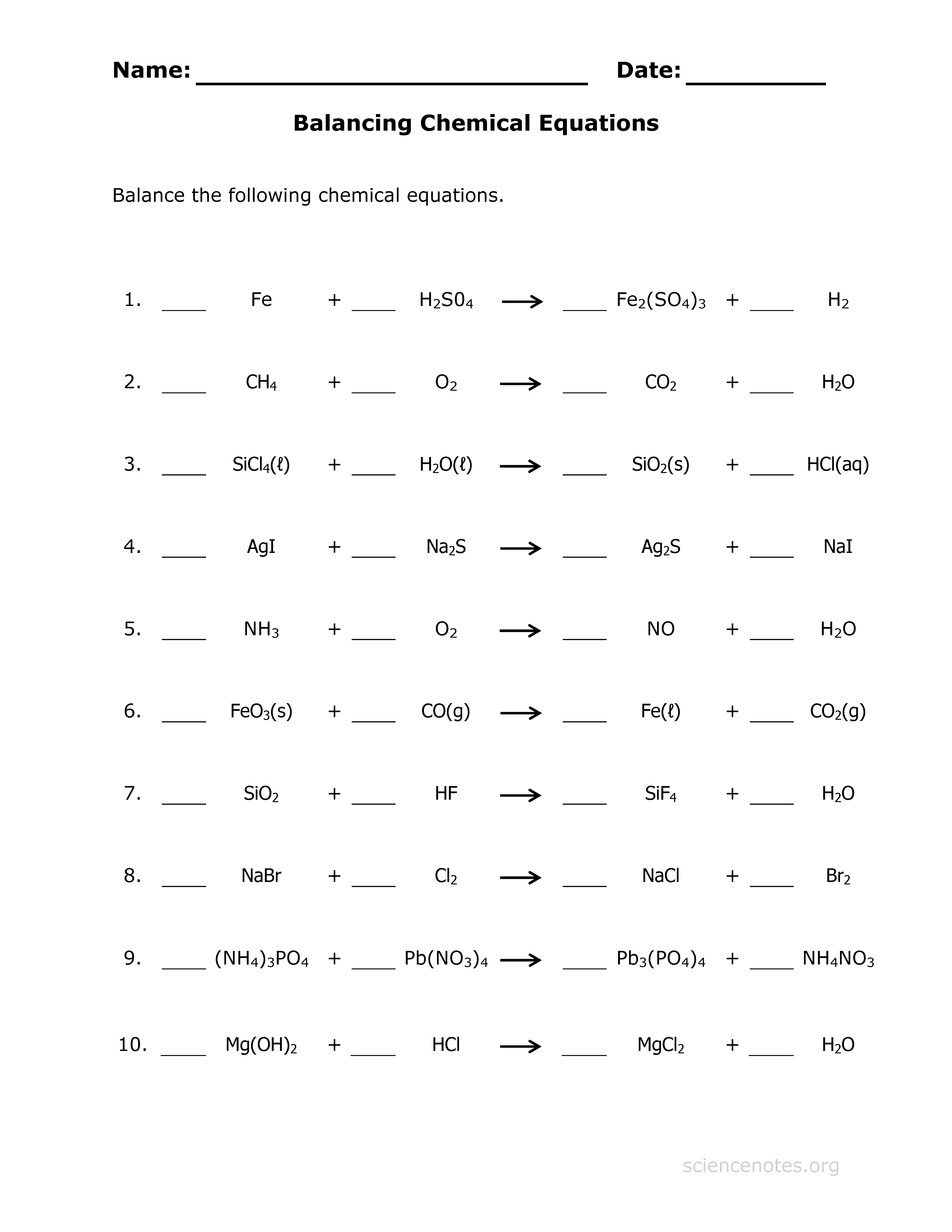
Free Chemistry Practice Worksheet

To put your knowledge to the test, here’s a free worksheet with several equations for balancing:
1. H2 + O2 → H2O
2. C3H8 + O2 → CO2 + H2O
3. Al + H2SO4 → Al2(SO4)3 + H2
4. FeCl3 + KOH → KCl + Fe(OH)3
5. P + O2 → P2O5
Try balancing these equations on your own. After you're done, you can check the answers at the end of this post.
Tips for Efficient Balancing
- Start with the most complex molecule to simplify the process.
- Balance elements that appear in only one reactant and product first.
- Save oxygen and hydrogen for last as they often appear in multiple compounds.
- Use the trial and error method when no clear path is evident.
- Remember that polyatomic ions can often be treated as units for balancing.
This guide and worksheet are intended to help you become proficient in balancing chemical equations. By understanding the core concepts and practicing with various examples, you'll improve your skills in chemistry, making complex reactions and calculations more manageable.
Why do we balance chemical equations?

+
We balance chemical equations to satisfy the law of conservation of mass, ensuring that matter is neither created nor destroyed in a chemical reaction.
What if my equation still doesn’t balance after using all coefficients?

+
If your equation remains unbalanced, recheck your work or consider that there might be other substances involved (like water or gases like O2) that need to be considered.
Can chemical equations be balanced by changing subscripts?

+
No, changing subscripts alters the identity of compounds. Use only coefficients to balance the equation.