Intramolecular vs Intermolecular Forces: Worksheet Answers Explained
Intramolecular forces and intermolecular forces are foundational concepts in chemistry that explain how atoms and molecules interact with each other. Understanding these forces is crucial for both academic learning and real-world applications, ranging from understanding boiling points to molecular biology. This article aims to clarify these concepts by providing detailed explanations and examples through a worksheet's answers.
What are Intramolecular Forces?

Intramolecular forces, often referred to as chemical bonds, are the forces of attraction that hold atoms together within a molecule. These forces are considerably stronger than the forces between molecules:
- Ionic bonds: Form when one or more electrons are transferred from one atom to another, creating a cation and an anion.
- Covalent bonds: Involving the sharing of electron pairs between atoms, covalent bonds can be polar (unequal sharing) or non-polar (equal sharing).
- Metallic bonds: These bonds hold metal atoms together in a solid lattice, allowing for the conductivity and malleability of metals.
🔍 Note: Intramolecular forces are what make the molecule; they are strong enough to keep the molecule intact under normal conditions.
What are Intermolecular Forces?

Intermolecular forces are weaker than intramolecular forces and are the forces of attraction between different molecules. They include:
- London Dispersion Forces (LDF): These are temporary dipoles caused by the movement of electrons within a molecule, leading to a weak attraction between neutral molecules.
- Dipole-Dipole Interactions: These forces exist between the positive end of one polar molecule and the negative end of another.
- Hydrogen Bonds: A special case of dipole-dipole interactions, where hydrogen is bonded to nitrogen, oxygen, or fluorine, resulting in a particularly strong interaction.
Here is a table comparing the strengths of these forces:
| Force Type | Strength |
|---|---|
| Ionic Bond | High |
| Covalent Bond | High |
| Metallic Bond | Moderate-High |
| Hydrogen Bond | Moderate |
| Dipole-Dipole | Low-Moderate |
| London Dispersion Forces | Low |

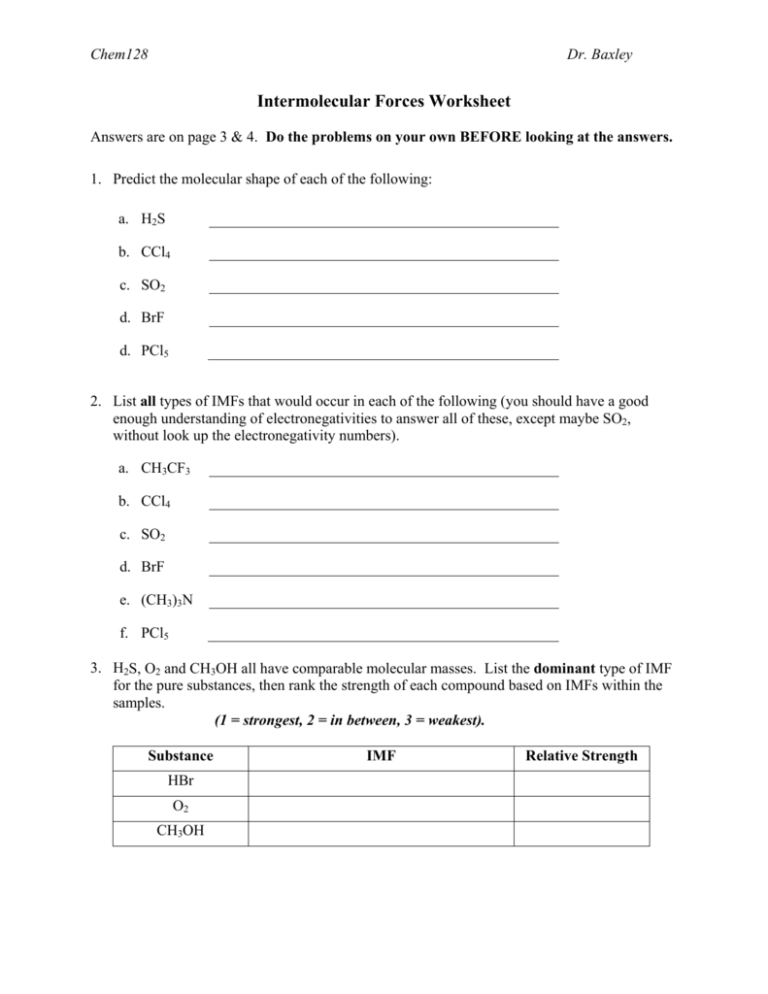
Worksheet Questions and Answers

Question 1: Difference between Intramolecular and Intermolecular Forces

Explain the primary differences between intramolecular and intermolecular forces.
Answer: Intramolecular forces are the forces that bond atoms within a molecule, making it stable and having a defined shape. Intermolecular forces, on the other hand, are the weak attractions between different molecules, influencing properties like boiling and melting points but not the molecule’s internal structure.
Question 2: Example of Intermolecular Forces

Provide examples of intermolecular forces in common substances.
Answer:
- Water (H2O): Hydrogen bonding allows water to have a high boiling point and surface tension.
- Ammonia (NH3): Similar to water, ammonia forms hydrogen bonds due to the electronegativity of nitrogen.
- Hydrocarbons like methane (CH4) are mainly influenced by London Dispersion Forces because of their non-polar nature.
💧 Note: The presence of hydrogen bonds in water explains many of its unique properties, such as its ability to dissolve many substances.
Question 3: Effects of Intermolecular Forces on Physical Properties

Discuss how intermolecular forces affect the physical properties of substances.
Answer: Intermolecular forces have a significant impact on:
- Boiling Point: Stronger intermolecular forces mean higher energy is required to separate the molecules, leading to higher boiling points. Water, for instance, has a high boiling point due to hydrogen bonds.
- Melting Point: Similar to boiling point, substances with stronger intermolecular forces require more energy to transition from solid to liquid, resulting in higher melting points.
- Viscosity: Substances with stronger intermolecular forces often have higher viscosity as the molecules resist flowing past one another.
- Solubility: Polar solvents dissolve polar solutes due to attractive forces between the molecules.
Question 4: Comparison of Covalent and Ionic Bonds

Compare and contrast covalent and ionic bonds.
Answer:
| Covalent Bonds | Ionic Bonds |
|---|---|
| Electrons are shared between atoms. | Electrons are transferred, creating oppositely charged ions. |
| Typically found in non-metals bonding with non-metals. | Commonly between metals and non-metals. |
| Can be polar or non-polar. | Always polar due to the complete electron transfer. |
| Molecules formed have specific shape and structure. | Ionic compounds form crystal lattices. |
In essence, this worksheet has demonstrated how intramolecular and intermolecular forces play distinct yet interconnected roles in the physical and chemical properties of substances. From the strength of ionic bonds to the subtleties of London Dispersion Forces, understanding these forces allows for a deeper comprehension of chemistry's fundamental principles. The table comparing covalent and ionic bonds further elucidates the differences between these intramolecular forces, which are essential for forming stable compounds.
Understanding these forces not only aids in academic study but also has practical implications in fields like pharmaceuticals, where drug design often leverages intermolecular interactions, or in materials science, where molecular structure and interactions determine the properties of new materials.
As we conclude, remember that the essence of chemistry often revolves around the interactions at the molecular level. Whether it's predicting how a substance will behave under different conditions or designing new substances with specific properties, intramolecular and intermolecular forces are your guide to understanding the physical world at its most fundamental level.
What is the strongest type of chemical bond?

+
Covalent bonds are typically the strongest type of chemical bond due to the electron sharing between atoms. However, metallic bonds in some transition metals can also be quite strong.
Why does water have such unique properties?

+
Water’s unique properties stem from hydrogen bonding, where the polarity of water molecules allows them to form strong intermolecular attractions, influencing boiling point, surface tension, and its ability to dissolve many substances.
How do intermolecular forces affect the vapor pressure of a substance?

+
The stronger the intermolecular forces, the lower the vapor pressure at a given temperature, because it requires more energy to overcome these forces for the molecules to escape into the vapor phase.



