Balancing Equations Made Easy: Worksheet Answer Key

Balancing chemical equations can often seem like a complex task, but with the right approach, it becomes straightforward and manageable. This comprehensive guide will not only walk you through the step-by-step process of balancing chemical equations but also provide you with detailed worksheet answer keys to help you master this fundamental concept in chemistry.
What is a Balanced Chemical Equation?

A balanced chemical equation represents a chemical reaction where the number of atoms for each element in the reactants equals the number in the products. Here are key points to understand:
- Law of Conservation of Mass: Matter cannot be created or destroyed in a chemical reaction.
- Balancing ensures that the total mass of the reactants equals the total mass of the products.
Steps to Balance Chemical Equations

1. Identify the Reactants and Products

Start by listing out each compound or element on both the reactants and products side of the equation. Here’s an example:
[2H_2 + O_2 \rightarrow 2H_2O]
2. Count the Number of Atoms

Count the atoms of each element:
| Element | Reactants | Products |
|---|---|---|
| Hydrogen (H) | 4 | 4 |
| Oxygen (O) | 2 | 2 |

3. Begin Balancing

- Adjust coefficients to balance the number of atoms. Use the smallest integers possible.
- Balance elements that appear in the least number of formulas first.
📌 Note: When balancing, do not alter the subscripts within the chemical formulas.
4. Verify the Balance

Once you’ve adjusted the coefficients, check if the number of atoms for each element is the same on both sides:
- Hydrogen: 4 (Reactants) = 4 (Products)
- Oxygen: 2 (Reactants) = 2 (Products)
5. Finalize the Equation

Ensure all coefficients are in the simplest whole number ratio. The balanced equation should look like this:
[ 2H_2 + O_2 \rightarrow 2H_2O ]
Example Worksheet Answer Key
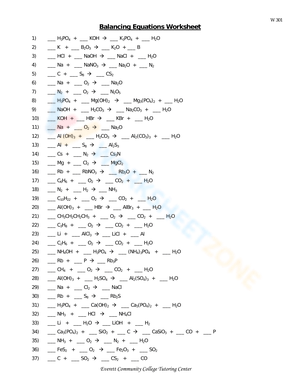
Here are some common chemical reactions and their balanced equations:
- Combustion of Methane:
- Reaction of Sodium with Water:
- Decomposition of Potassium Chlorate:
\[ CH_4 + 2O_2 \rightarrow CO_2 + 2H_2O \]
\[ 2Na + 2H_2O \rightarrow 2NaOH + H_2 \]
\[ 2KClO_3 \rightarrow 2KCl + 3O_2 \]
✅ Note: Balancing equations often require trial and error. It's common to make several attempts before finding the correct balance.
Advanced Balancing Techniques

For more complex reactions, here are some additional strategies:
- Using the Method of Odd-Even Numbers: If you encounter an odd number of atoms on one side and even on the other, balance the odd side first.
- Fractional Coefficients: In some cases, fractional coefficients can be used temporarily to achieve balance, but the final equation should always have whole numbers.
After understanding these strategies, balancing complex equations like photosynthesis or reactions involving polyatomic ions becomes more manageable.
In conclusion, by following a systematic approach, balancing chemical equations can be demystified. The key is to start with the basics, understand the logic behind the process, and practice with various types of reactions. With these techniques, not only will you become proficient in balancing equations, but you'll also gain a deeper understanding of the chemical processes at play.
Why is it important to balance chemical equations?

+
Balancing chemical equations ensures that the chemical reaction adheres to the law of conservation of mass, meaning no atoms are created or destroyed during the reaction.
Can you change the subscripts while balancing an equation?

+
No, you cannot change the subscripts. Subscripts define the molecular formula of a compound, and altering them would change the identity of the compound.
What to do if balancing an equation results in a large coefficient?

+
If an equation results in a large coefficient, you might try to simplify it by dividing all coefficients by their greatest common divisor to get the simplest whole number ratio.
How do you know when you’ve correctly balanced an equation?

+
You know you’ve balanced an equation correctly when the number of atoms for each element on both the reactants and products side are equal.
Are there computer programs for balancing equations?

+
Yes, there are numerous online tools and educational software that can help you balance chemical equations quickly and accurately.



