5 Tips for Mastering Balance Chemistry Equations

Understanding Chemical Equations

Before diving into the art of balancing chemical equations, it’s crucial to understand what they represent. Chemical equations show reactants transforming into products. For example, in the simple combustion of methane:
[ CH_4 + O_2 \rightarrow CO_2 + H_2O ]
We can see methane (CH4) and oxygen (O2) reacting to form carbon dioxide (CO2) and water (H2O). Balancing these equations involves adjusting the coefficients to ensure the number of atoms on both sides of the equation is the same, adhering to the law of conservation of mass.
Tip 1: List All Atoms
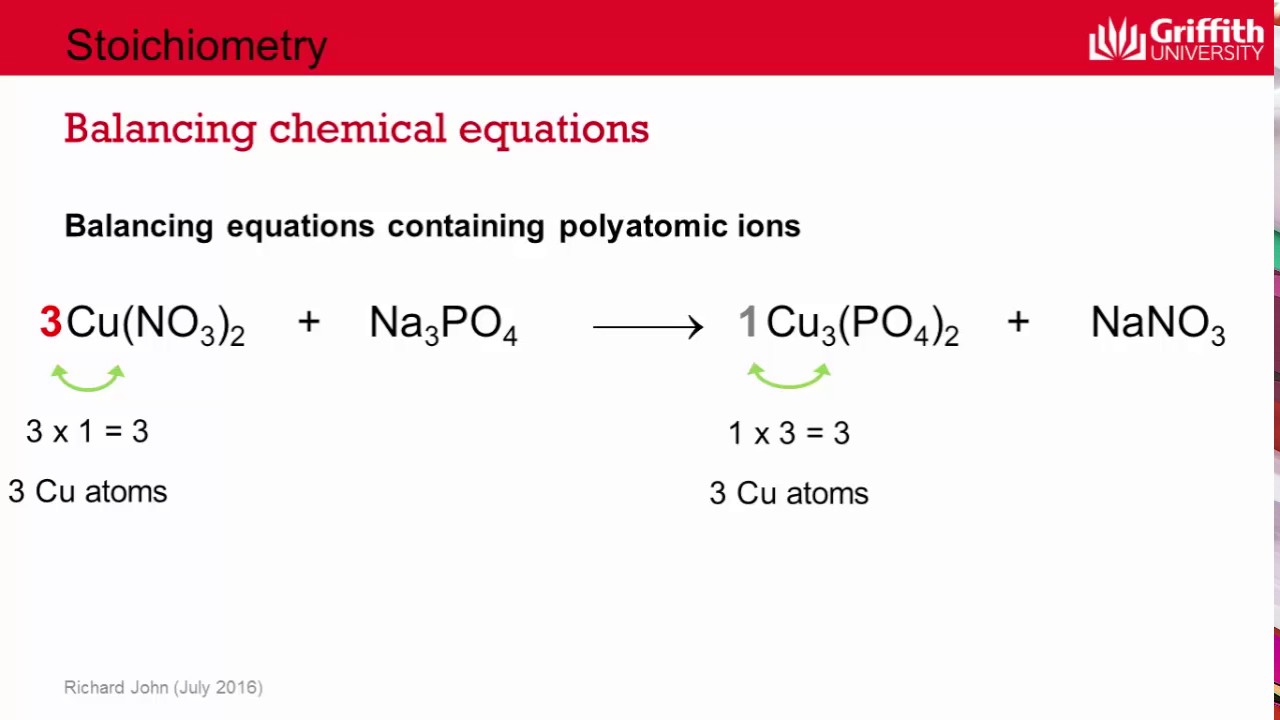
The first step in balancing an equation is listing all the atoms on each side:
- Reactants: Carbon © = 1, Hydrogen (H) = 4, Oxygen (O) = 2
- Products: Carbon © = 1, Hydrogen (H) = 2, Oxygen (O) = 3
This initial listing helps identify which atoms are in excess or deficit.
Tip 2: Identify the Balanced Atom

In our example, the Carbon © atoms are already balanced, so we can focus on the others. This approach reduces complexity and allows for a smoother balancing process:
[ CH_4 + O_2 \rightarrow CO_2 + 2H_2O ]
Tip 3: Use Coefficients, Not Subscripts
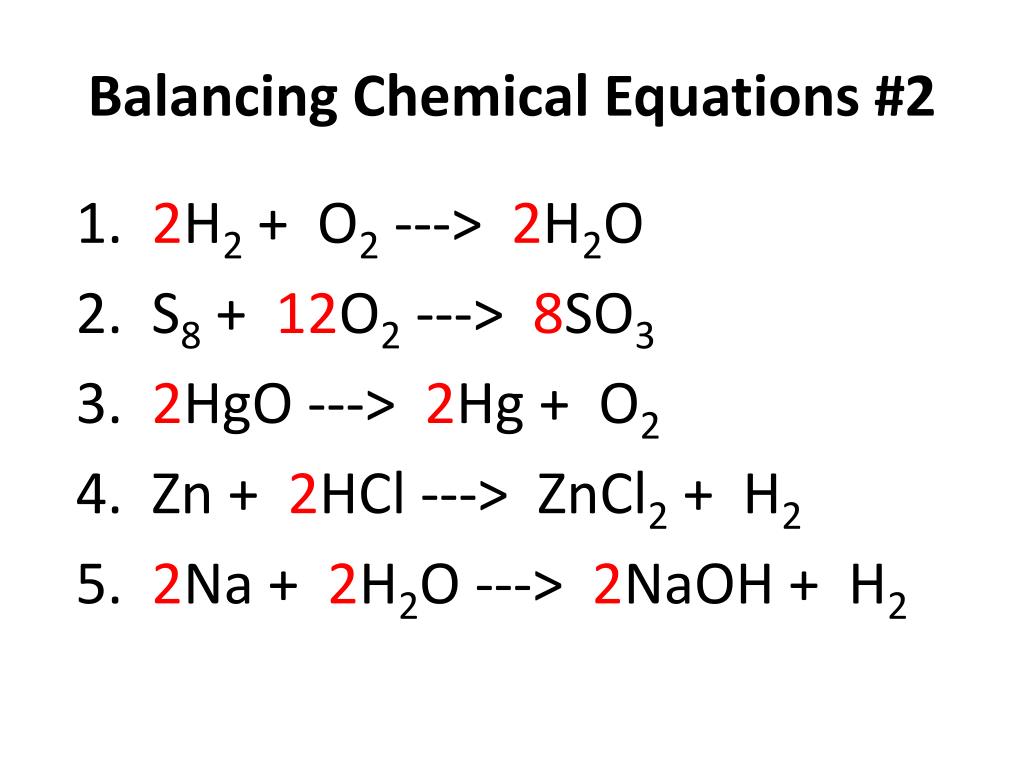
Balancing must be done by adjusting the coefficients, not the subscripts, as changing subscripts alters the chemical identity of the compounds:
- Incorrect: CH3 instead of CH4
- Correct: 2H2O instead of H2O
Tip 4: Use Fractions when Necessary
If you encounter difficulty balancing, use fractions as a temporary measure to find the least common denominator for coefficients:
For example, to balance the Oxygen atoms:
[ CH_4 + O_2 \rightarrow CO_2 + H_2O ]
We can start with:
[ CH_4 + 2O_2 \rightarrow CO_2 + 2H_2O ]
Now, the equation is temporarily balanced using a fraction:
[ CH_4 + 2.5O_2 \rightarrow CO_2 + 2H_2O ]
This equation can be balanced by multiplying everything by 2 to get rid of the fraction:
[ 2CH_4 + 5O_2 \rightarrow 2CO_2 + 4H_2O ]
⚠️ Note: Although fractions can be used in the process, all equations must end up with whole number coefficients for simplicity and accuracy.
Tip 5: Simplify if Possible

After balancing, always look to simplify the coefficients if possible:
| Original | Simplified |
|---|---|
| 2CH4 + 5O2 → 2CO2 + 4H2O | CH4 + 2.5O2 → CO2 + 2H2O (simplified by halving) |

💡 Note: Simplifying is optional but can make the equation easier to understand and work with.
The journey to mastering the art of balancing chemical equations is a process of patience, practice, and pattern recognition. Each equation presents a unique challenge, requiring a blend of logic and chemical intuition. Here's a brief summary of the key points:
- List Atoms: Identify and list all atoms involved in both reactants and products.
- Find Balance: Look for atoms that are already balanced to simplify the process.
- Adjust Coefficients: Use coefficients to ensure atom conservation, not subscripts.
- Fractional Balancing: Sometimes, using fractions as a temporary measure can lead to easier whole number solutions.
- Simplify: If possible, simplify the coefficients to make the equation more manageable.
Balancing equations is not just about ensuring that the numbers add up correctly; it's a testament to the fundamental principles of chemistry. This mastery can unlock a deeper understanding of chemical reactions, aiding students and scientists alike in their pursuit of knowledge.
Why is it important to balance chemical equations?
+
Balancing chemical equations is crucial because it adheres to the law of conservation of mass, which states that matter cannot be created or destroyed in a chemical reaction. This balance ensures that the reaction is stoichiometrically correct, meaning the quantities of reactants and products are precisely as they should be.
Can fractions be used in balancing equations?
+
Yes, fractions can be used as a temporary step to find the least common multiple for coefficients. However, the final equation should have whole number coefficients to reflect real-world amounts of substances.
What happens if I change the subscripts?

+
Changing the subscripts would alter the identity of the compounds, turning them into different substances. It’s not allowed in balancing equations as the goal is to balance quantities, not identities.
How do I know if an equation is balanced?

+
An equation is balanced when the number of each type of atom on the reactant side equals the number on the product side. You can use the list and compare method or inspection method to verify.