5 Essential Answers for Atoms, Ions, Isotopes Worksheet
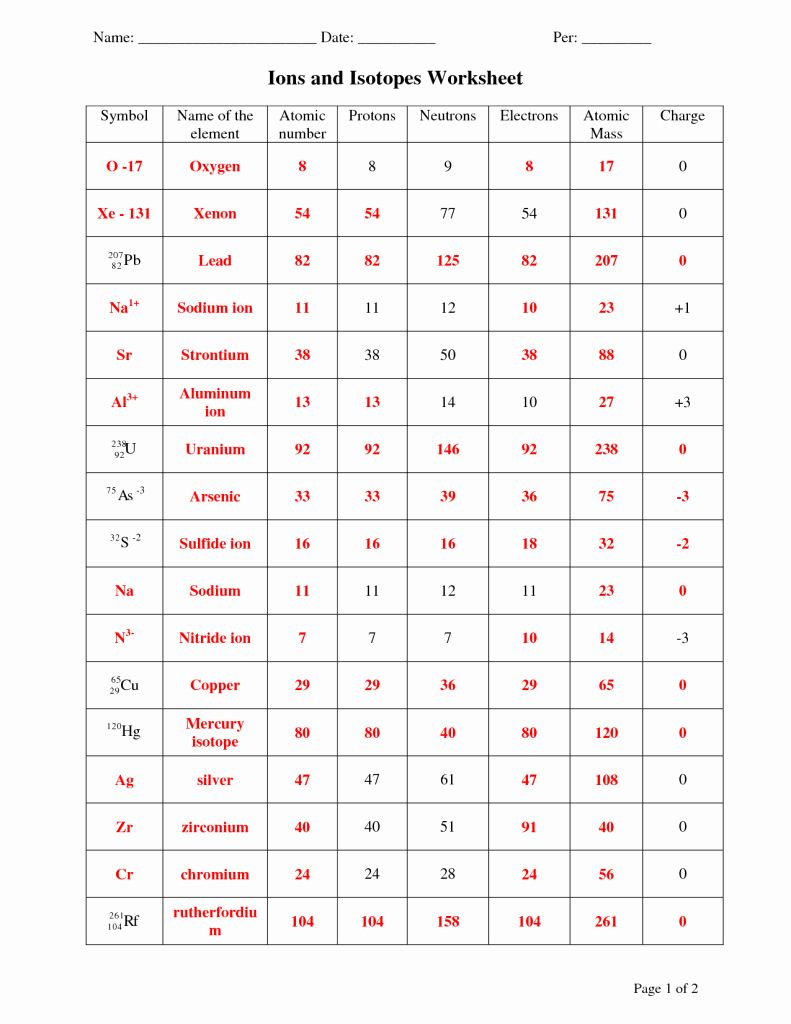
The concepts of atoms, ions, and isotopes form the bedrock of chemistry, making them essential for anyone diving into this intriguing science. Understanding these topics not only enhances your knowledge but also aids in comprehending how our world functions at the microscopic level. Here, we'll explore five essential answers related to these fundamental particles:
1. What are Atoms and Their Components?


At its core, an atom is the smallest unit of an element that retains the chemical properties of that element. It consists of three primary components:
- Nucleus: Found in the center of the atom, the nucleus contains:
- Protons: Positively charged particles with a mass of approximately 1 atomic mass unit (amu).
- Neutrons: Neutral particles with a similar mass to protons, also at about 1 amu.
- Electrons: These negatively charged particles orbit the nucleus in shells or energy levels, and their mass is roughly 1⁄1836 that of a proton or neutron. Electrons are crucial for chemical bonding as they interact to form compounds.
2. How Do Atoms Differ from Ions?

An atom becomes an ion when it gains or loses electrons, thereby changing its electrical charge:
- Cation: An ion that loses electrons to become positively charged.
- Anion: An ion that gains electrons, acquiring a negative charge.
⚠️ Note: The number of protons in an ion remains unchanged from the original atom, which distinguishes it from an isotope, where the neutron count can differ.
3. Explaining Isotopes

Isotopes are atoms of the same element with the same number of protons but different numbers of neutrons:
- This difference in neutrons leads to variations in atomic mass but not in chemical behavior since the number of protons defines the element.
- Some isotopes are stable, while others are radioactive and decay over time.
4. Identifying Elements and Isotopes

To identify an element or its isotope:
- Count the number of protons to determine the element. For example, an element with 6 protons is carbon.
- The sum of protons and neutrons gives the mass number. Isotopes will have different mass numbers.
| Element | Atomic Number | Mass Number | Isotope Notation |
|---|---|---|---|
| Carbon | 6 | 12 | 12C |
| Carbon | 6 | 14 | 14C |

5. Understanding Atomic Theory and Subatomic Particles

Atomic theory has evolved from Dalton’s model to the modern quantum mechanical view:
- Dalton’s Theory: Atoms are indivisible and indestructible.
- Thomson Model: Atoms have a positive fluid with embedded electrons.
- Rutherford Model: Centralized nucleus with orbiting electrons.
- Bohr Model: Electrons in fixed orbits around the nucleus.
- Quantum Mechanics: Electrons described by probability clouds rather than orbits.
📝 Note: This evolution reflects how our understanding of atomic structure has become more nuanced and complex over time.
In summary, atoms are the smallest units of elements, ions are charged atoms, and isotopes are different versions of the same element with varying neutron counts. This knowledge is not just academic; it underpins many chemical and physical phenomena observed in everyday life. Understanding these basics provides a foundation for exploring more complex chemical reactions and atomic interactions, making you better prepared for any chemistry coursework or real-world applications involving atomic theory.
What is the difference between an atom and an ion?

+
An atom is the basic unit of matter that has the properties of a chemical element. An ion, on the other hand, is an atom that has either lost or gained electrons, thus acquiring a net electric charge.
Can an isotope change into an ion?

+
Yes, isotopes, like any atom, can form ions by losing or gaining electrons. The isotope identity doesn’t change, but the atom becomes an ion due to the change in electron count.
How do isotopes affect atomic mass?

+
Isotopes with different numbers of neutrons will have different atomic masses. However, the atomic weight listed on periodic tables is often an average of all the naturally occurring isotopes of that element.



