Atomic Theory Timeline: Essential History Worksheet

Delving into the realm of science, the atomic theory stands as one of the most pivotal concepts in modern chemistry. This theory has undergone a rich historical evolution, marking significant milestones through contributions from numerous scientists. Let's explore the essential history of atomic theory through a detailed timeline.
The Ancient Beginnings


The very concept of atoms, or “atomos” in Greek, meaning indivisible, was first introduced by Greek philosophers. Around 450 B.C., Leucippus and his student Democritus proposed the idea that matter is composed of small, indivisible particles. While this was philosophical rather than scientific, it laid the groundwork for later theories.
John Dalton’s Modern Theory

In 1803, John Dalton published his atomic theory, which marked the beginning of its modern form:
- Elements are made of tiny particles called atoms.
- All atoms of a given element are identical in mass and properties.
- Compounds are formed when atoms of different elements combine in simple, whole number ratios.
- Chemical reactions involve the rearrangement of atoms; atoms themselves are not created or destroyed.
Significance

Dalton’s theory provided a scientific foundation for understanding the composition of matter and explaining the laws of chemical combination.
The Discovery of the Electron
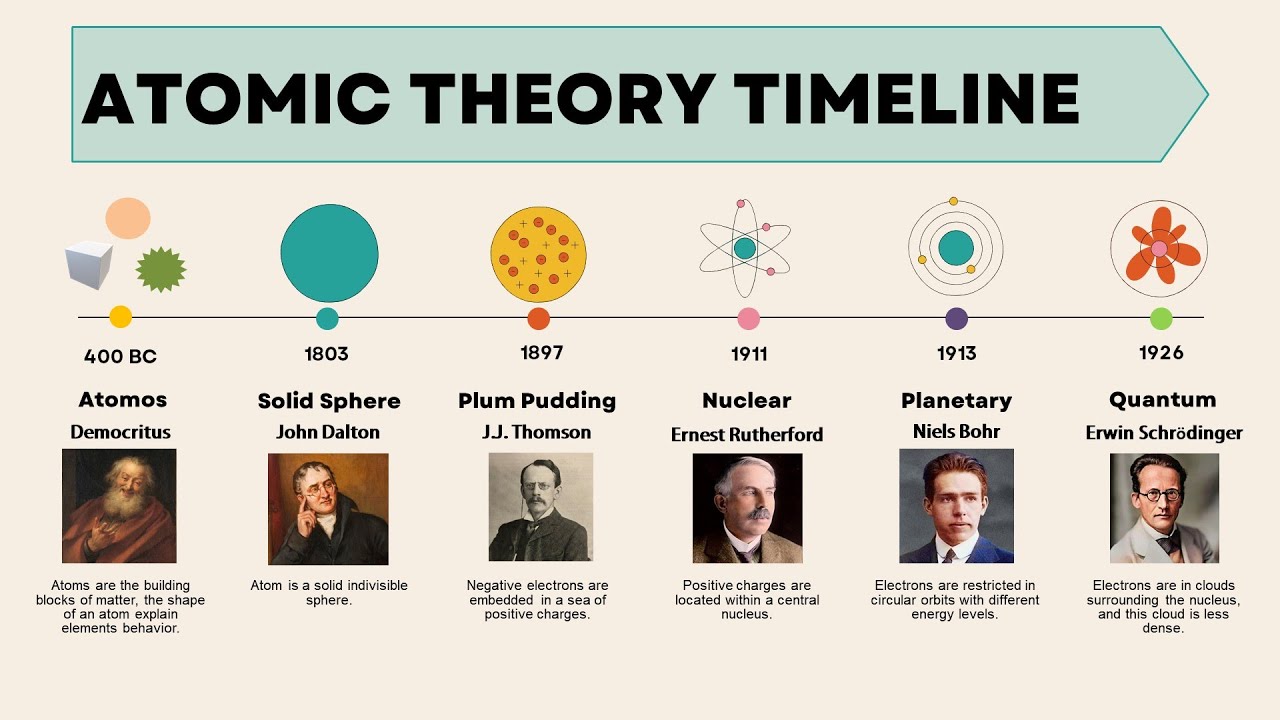
In 1897, J.J. Thomson discovered the electron through his experiments with cathode rays. This discovery:
- Showed that atoms were not indivisible as they contain smaller particles.
- Led to the “plum pudding model,” where electrons are embedded in a positively charged matrix.
The Nuclear Model


The atom’s internal structure was revolutionized by Ernest Rutherford in 1911:
- Using the alpha particle scattering experiment, he deduced that atoms have a dense, positively charged nucleus.
- This led to the rejection of Thomson’s model and the proposal of the nuclear model of the atom.
Niels Bohr’s Quantum Model

Niels Bohr refined the atomic model in 1913 by introducing quantum theory:
- He proposed that electrons orbit the nucleus in specific, quantized energy levels.
- This model explained the spectral lines of hydrogen and introduced the concept of electron shells.
Impact

Bohr’s model was instrumental in explaining atomic stability and why atoms emit or absorb energy at certain wavelengths.
Modern Atomic Theory


Over the 20th century, contributions from various scientists have shaped our current understanding of atoms:
- Wolfgang Pauli’s exclusion principle which dictates the arrangement of electrons in atoms.
- The discovery of the neutron by James Chadwick in 1932, providing the final piece of the atomic puzzle.
- The development of quantum mechanics which described the behavior of subatomic particles.
Key Points

| Scientist | Contribution | Year |
|---|---|---|
| Wolfgang Pauli | Exclusion Principle | 1925 |
| James Chadwick | Discovery of Neutron | 1932 |
| Erwin Schrödinger | Schrödinger Equation | 1926 |

🔬 Note: These contributions were critical in defining the modern atomic model which no longer views the atom as a simple structure but as a complex system governed by quantum mechanics.
By summarizing this journey from the ancient philosophy to the cutting-edge science of today, we witness the metamorphosis of an idea into an intricate understanding of the fundamental building blocks of nature. From Democritus to Schrödinger, each scientist has left an indelible mark on how we perceive matter and the universe.
Why is John Dalton’s contribution considered a cornerstone in atomic theory?

+
John Dalton provided the first scientific, rather than philosophical, theory of the atom, setting the stage for further scientific inquiries into the nature of matter.
How did the discovery of the electron change our understanding of atoms?

+
The discovery of the electron revealed that atoms are not indivisible but are composed of even smaller particles, fundamentally altering our view of atomic structure.
What is the significance of Niels Bohr’s model?

+
Bohr’s model integrated quantum theory with atomic structure, explaining the spectral lines of hydrogen and introducing the concept of discrete energy levels for electrons.
What are the key differences between Dalton’s and modern atomic theories?

+
Dalton’s theory viewed atoms as solid, indivisible units. Modern theory acknowledges atoms as complex structures with a nucleus and orbiting electrons, shaped by quantum mechanics.
How does quantum mechanics relate to atomic theory?

+
Quantum mechanics describes the probabilistic nature of electron positions and behaviors within atoms, offering a more accurate depiction than classical models could provide.