Atomic Structure Worksheet Answers: Inside Atom Secrets Revealed

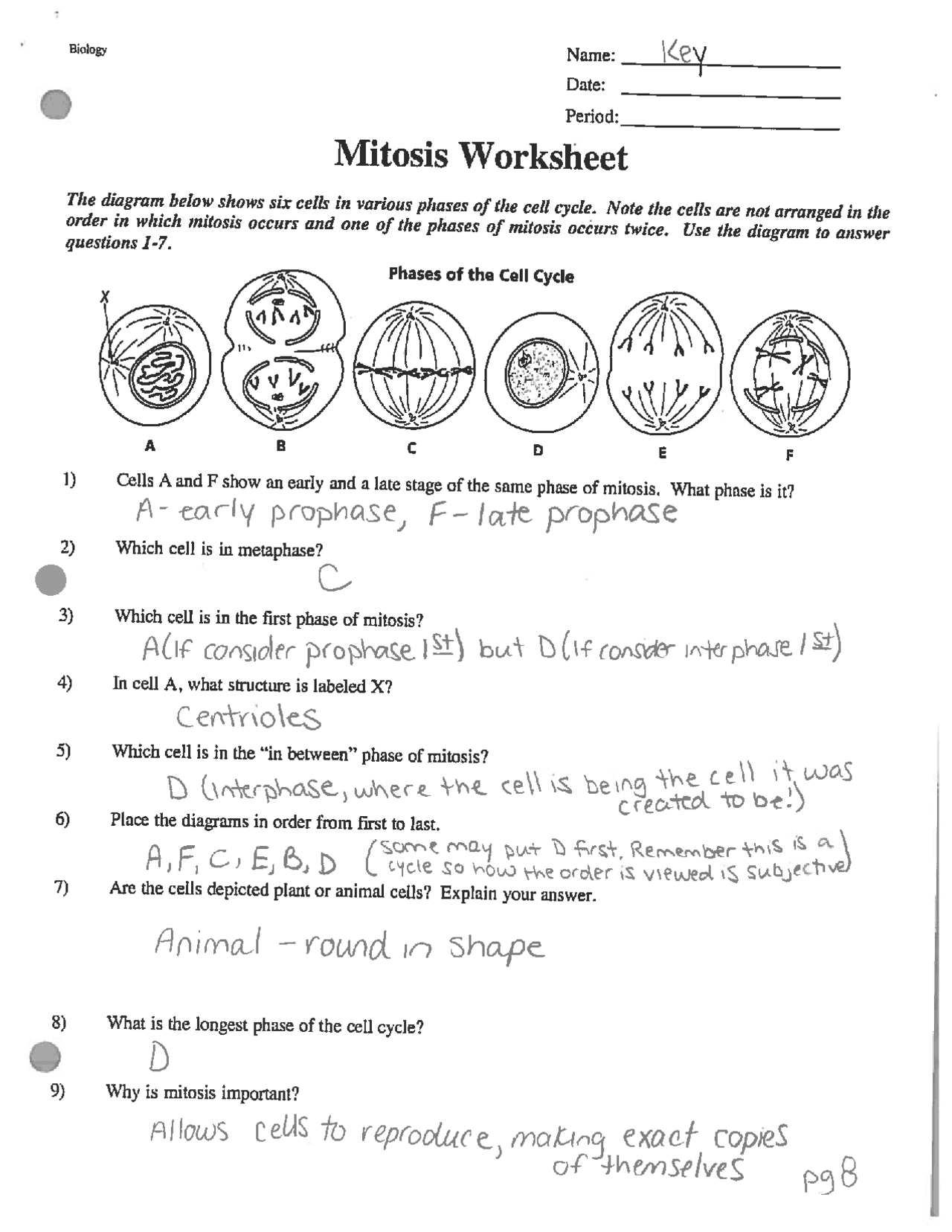
Understanding the intricate world of atoms can sometimes feel like trying to solve a complex puzzle. At the heart of chemistry lies the atomic structure, a fundamental concept that describes how matter is built at the most basic level. This blog post will delve deep into atomic structure, providing a comprehensive resource for students, educators, and chemistry enthusiasts looking to explore or clarify this topic. Through detailed explanations, step-by-step answers to common worksheet questions, and insightful notes, we'll uncover the secrets hidden within atoms.
The Basics of Atomic Structure

An atom is the smallest unit of an element that retains the chemical properties of that element. Here are the key components:
- Protons: Positively charged particles found in the nucleus of an atom.
- Electrons: Negatively charged particles that orbit the nucleus.
- Neutrons: Neutral particles located in the nucleus, which contribute to the mass of the atom but not its charge.
Elementary Particles and Their Properties
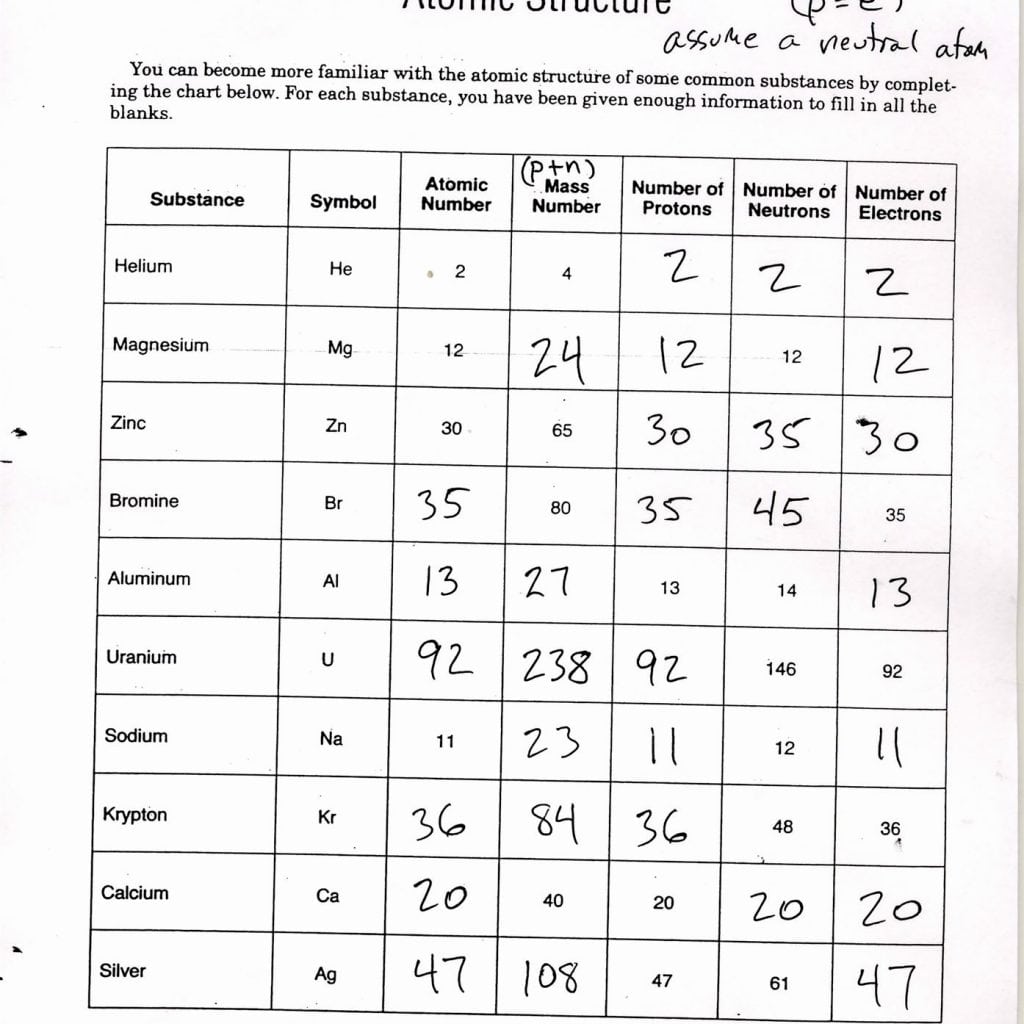
The table below outlines the properties of these elementary particles:
| Particle | Charge | Location | Mass (amu) |
|---|---|---|---|
| Proton | +1 | Nucleus | 1.0073 |
| Electron | -1 | Orbits Nucleus | 0.0005485799 |
| Neutron | 0 | Nucleus | 1.0087 |

📝 Note: The atomic mass unit (amu) is defined as one-twelfth the mass of a carbon-12 atom.
The Electron Configuration

One of the key elements in understanding atomic structure is how electrons are arranged around the nucleus:
- Electrons are arranged in shells or energy levels around the nucleus.
- Each shell can contain a certain number of electrons:
- First shell: Up to 2 electrons
- Second shell: Up to 8 electrons
- Third shell: Up to 18 electrons, but usually only 8 due to stability
- The aufbau principle, Hund’s rule, and the Pauli exclusion principle guide electron configuration.
Atomic Models Through Time

From Dalton’s solid sphere model to Bohr’s planetary model, and quantum mechanics:
- Dalton’s Model: Atoms were considered as indivisible spheres.
- Thomson’s Plum Pudding Model: Atoms contain negatively charged electrons scattered in a positively charged “pudding.”
- Rutherford’s Nuclear Model: Atoms have a dense central nucleus with orbiting electrons.
- Bohr’s Model: Electrons occupy specific orbits with fixed energies.
- Quantum Mechanical Model: Electrons exist in probability clouds known as orbitals.
🔬 Note: These models represent a progression in our understanding of atomic structure, each building upon the last.
Common Worksheet Questions Explained

Here are answers to some typical questions found in atomic structure worksheets:
1. How many protons, neutrons, and electrons are in an atom of Oxygen-16?

Oxygen-16 has:
- 8 protons (from periodic table)
- 8 neutrons (16-8 = 8)
- 8 electrons (equal to the number of protons in a neutral atom)
2. What does the mass number signify in an atomic symbol?
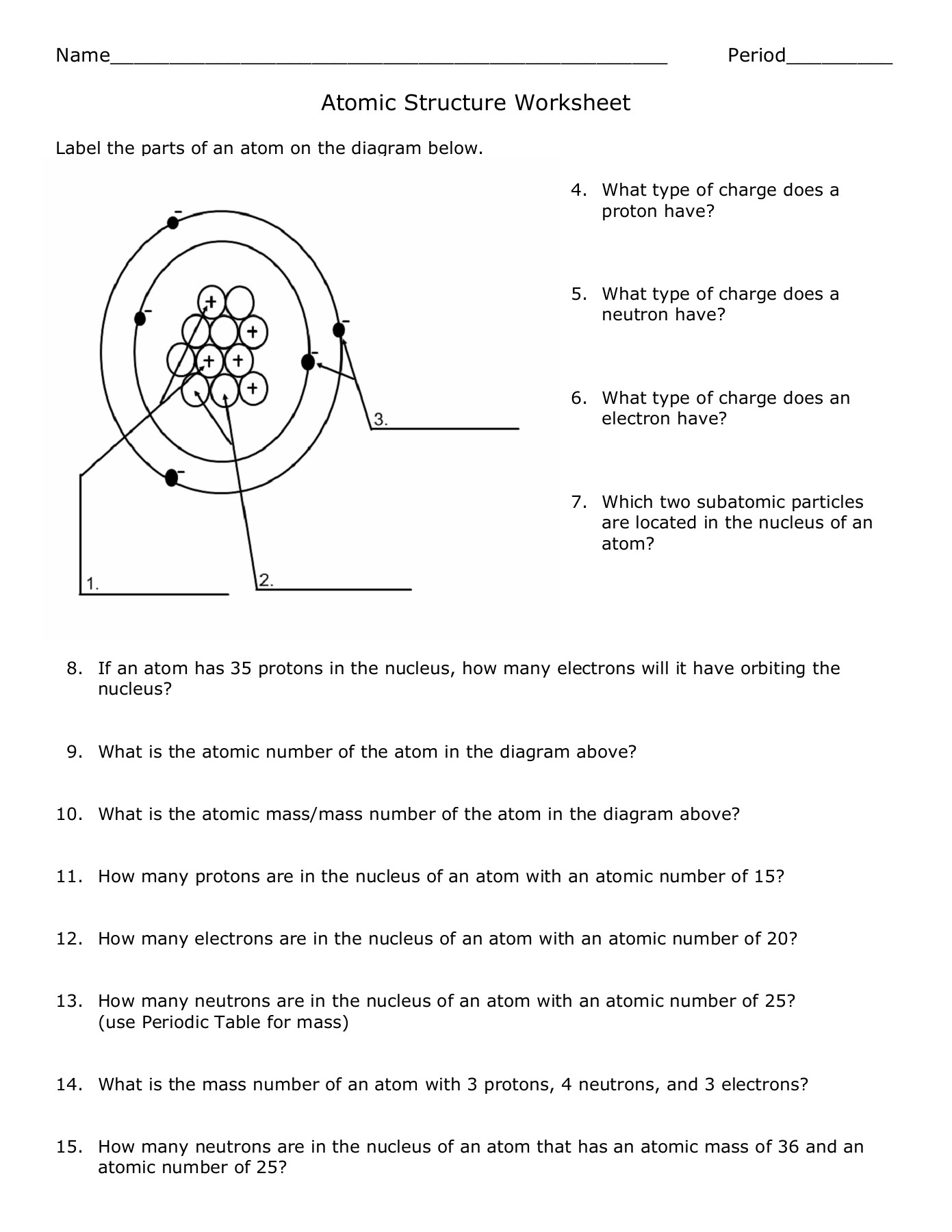
The mass number (the superscript in an atomic symbol) represents the total number of protons and neutrons in the nucleus of an atom.
3. Explain the concept of Isotopes

Isotopes are variants of a chemical element with the same number of protons but different numbers of neutrons. This results in different mass numbers but similar chemical properties.
4. How can you determine the number of valence electrons from an element’s position on the periodic table?

The number of valence electrons can often be inferred from an element’s group number on the periodic table:
- Group 1 (alkali metals) have 1 valence electron.
- Group 18 (noble gases) generally have 8 valence electrons (with exceptions like helium having 2).
- Elements in other groups follow similar patterns, adjusting for the octet rule.
💡 Note: Valence electrons are crucial as they participate in chemical reactions and bonding.
Conclusion

In this detailed exploration, we’ve unraveled the secrets of the atomic structure. From understanding the basic constituents of an atom to delving into how electrons are arranged, we’ve covered essential concepts that underpin much of modern chemistry. Whether you’re a student grappling with atomic structure worksheets or an enthusiast looking to deepen your knowledge, this post serves as a guide to better comprehend the building blocks of matter. The transition through various atomic models showcases the evolving nature of scientific knowledge, each new model refining our understanding of atoms.
What is the importance of neutrons in an atom?

+
Neutrons help stabilize the nucleus by balancing the electrical repulsion between protons with their strong nuclear force, aiding in the atom’s stability.
How does the atomic number affect an element’s identity?

+
The atomic number is the number of protons in an atom’s nucleus, which defines the element’s chemical identity, as no two elements have the same number of protons.
Why do atoms form bonds?

+
Atoms form bonds to achieve stability, usually by filling their valence shell to the most stable electron configuration, following the octet rule or achieving a noble gas configuration.
Can you have an atom without neutrons?

+
Yes, elements like hydrogen-1 (protium) have no neutrons, but it’s rare, and such atoms are less stable due to the lack of neutron interaction to stabilize the nucleus.