7 Essential Answers for Atomic Structure Worksheet
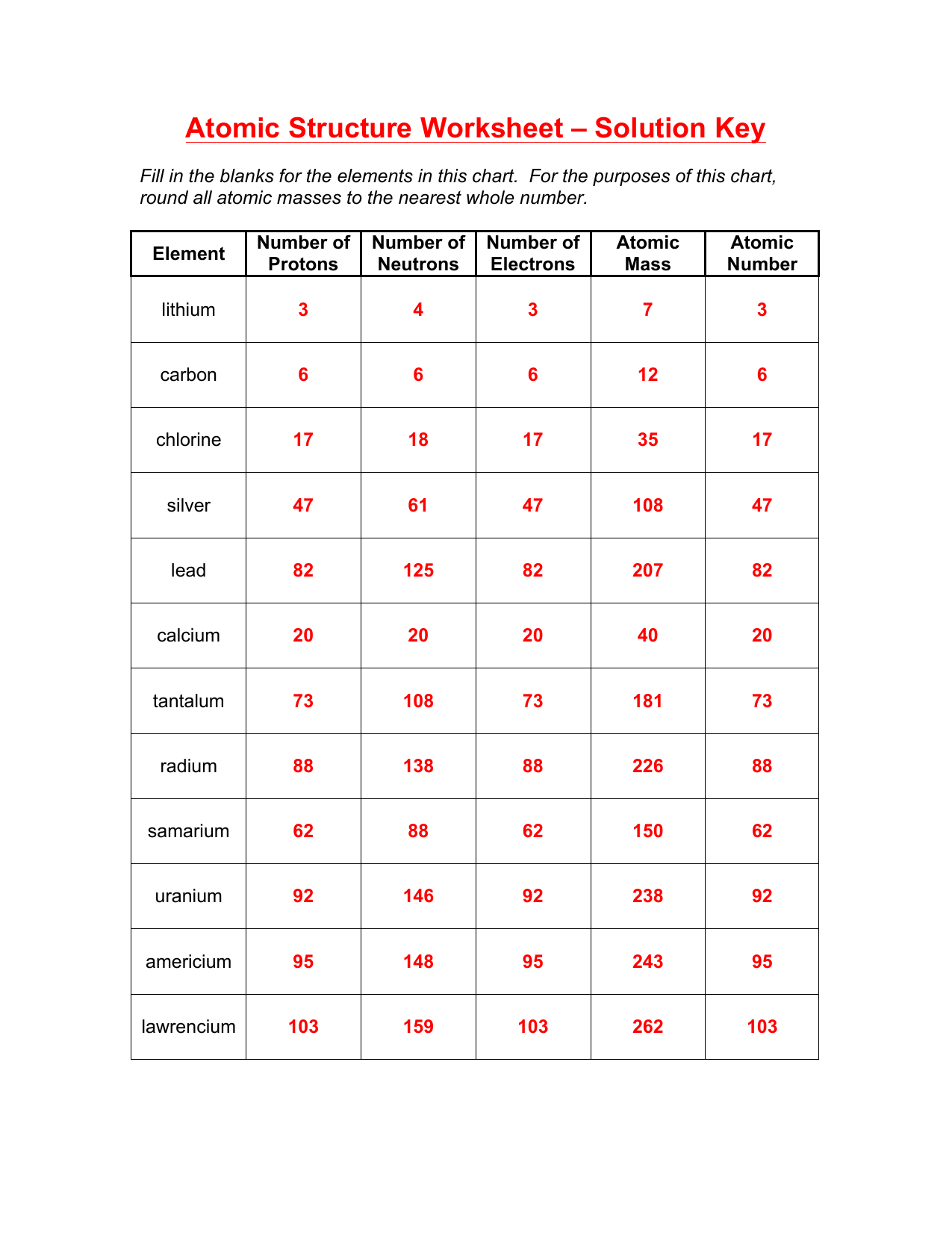
Understanding atomic structure is crucial for students studying chemistry, as it forms the foundation for all chemical reactions and bonding. Atomic structure worksheets are popular tools used to reinforce learning about the atom's building blocks - protons, neutrons, electrons, and the arrangement of electrons in different energy levels. Let's delve into seven essential answers that clarify key concepts in atomic structure.
The Basics of Atomic Structure

Atoms, the fundamental units of matter, consist of three primary subatomic particles:
- Protons - Positively charged particles found in the nucleus of the atom.
- Neutrons - Neutral particles, also found in the nucleus.
- Electrons - Negatively charged particles orbiting the nucleus in various energy levels or shells.
Understanding these components is the first step in mastering atomic structure.
What is the Difference Between Atomic Number and Mass Number?

The atomic number is the number of protons in an atom's nucleus, determining the element's identity. For instance, all hydrogen atoms have an atomic number of 1. On the other hand, the mass number represents the sum of protons and neutrons in an atom's nucleus. This means that different isotopes of an element can have different mass numbers due to variations in neutron counts.
How do Electron Configurations Work?

Electron configurations describe the distribution of electrons in an atom’s orbitals. Here's how they work:
- The electrons are arranged in energy levels, also known as shells, labeled by numbers (1, 2, 3...).
- Within each shell, there are sub-shells labeled s, p, d, f, etc.
- The configuration follows the Aufbau principle, where electrons fill lower-energy orbitals before filling higher-energy ones.
An example would be the electron configuration of carbon, which is 1s² 2s² 2p², indicating:
- Two electrons in the first shell (1s²).
- Four electrons in the second shell: two in the s orbital (2s²) and two in the p orbitals (2p²).
What is an Isotope?

An isotope refers to variants of an element that have the same number of protons but different numbers of neutrons. Here are the essential points about isotopes:
- Isotopes have the same atomic number but different mass numbers.
- They can be represented as:
- Isotopes exhibit different physical properties but mostly the same chemical behavior due to having the same number of electrons.
| Element Symbol | Mass Number (Superscript) |
|---|---|
| C | 12, 13, 14, etc. |

What Does the Periodic Table Tell Us About Atomic Structure?

The periodic table is an organized chart of elements, providing invaluable information about atomic structure:
- The rows (periods) reflect the energy levels of electrons, with each row filling a new shell.
- The columns (groups) indicate how many electrons are present in the outermost shell, which determines chemical behavior.
- Element blocks (s, p, d, f) indicate the last orbital that was filled.
Why is Atomic Radius Important?

Atomic radius is the size of an atom, which varies depending on the element. Here are reasons why atomic radius is significant:
- It influences how atoms interact with each other during bonding.
- The atomic radius increases as you move down a group due to the addition of electron shells.
- Conversely, it decreases across a period because electrons are added to the same shell, which increases the effective nuclear charge, pulling electrons closer to the nucleus.
How do Ions Relate to Atomic Structure?

Ions are atoms that have lost or gained electrons, resulting in a net electrical charge:
- Cations are positively charged due to losing electrons.
- Anions are negatively charged because they gain extra electrons.
- The formation of ions changes the electron configuration of an atom, often leading to a more stable electron arrangement.
⚗️ Note: The stability of ions is often related to the octet rule, which suggests that having eight electrons in the outer shell (for elements beyond neon) provides a stable electronic configuration.
In wrapping up these key answers, it's evident that understanding atomic structure is pivotal for anyone delving into the world of chemistry. From the fundamental particles that compose atoms to the complex interactions these particles exhibit, each concept builds upon the last to form a coherent picture of how matter behaves at the smallest scales. Appreciation of these structures helps predict the chemical behavior of elements, the properties of compounds, and the mechanics of reactions. Gaining a solid foundation in atomic structure not only enhances one's academic pursuits but also fosters a deeper understanding of the world at the microscopic level.
How does the atomic number determine an element’s properties?

+
The atomic number represents the number of protons in an atom’s nucleus, which determines the element’s identity and its chemical behavior. For instance, elements in the same group have similar atomic numbers and, hence, similar outer electron configurations, leading to analogous chemical properties.
Can two different elements have the same number of neutrons?

+
Yes, isotopes of different elements can have the same number of neutrons. For example, Carbon-14 and Nitrogen-15 both have 7 neutrons, but their different proton counts define them as separate elements.
Why is the concept of electron configuration important?

+
Electron configuration dictates how atoms will bond, their chemical reactivity, and the types of compounds they will form. It provides a systematic way of understanding how electrons are distributed, which in turn influences an element’s behavior.
How does atomic size influence chemical reactions?

+
Atomic size affects how closely atoms can approach each other, which influences bond strengths, bond lengths, and the reactivity of elements. Smaller atoms can form stronger bonds because their electrons are closer to the nucleus, experiencing stronger electrostatic attractions.
What are valence electrons, and why are they significant?

+
Valence electrons are those in the outermost shell of an atom, and they are crucial for determining the atom’s ability to lose, gain, or share electrons with other atoms. The number and arrangement of valence electrons define how an atom will interact chemically with others.



