Master Alpha and Beta Decay with This Worksheet

Understanding the intricate dance of particles within atomic nuclei can be a daunting task, but with the right guidance, even the complex concepts of alpha and beta decay can be made clear. This blog post will take you through a comprehensive worksheet tailored for mastering these types of radioactive decay, ensuring you not only understand the theories but also apply them with confidence.
What is Radioactive Decay?
Radioactive decay is the process by which an unstable atomic nucleus loses energy by emitting particles. This process helps the nucleus to move towards a more stable configuration. Understanding radioactive decay is pivotal in fields like nuclear physics, chemistry, and even in practical applications such as carbon dating and nuclear medicine.
The Alpha Decay: A Closer Look

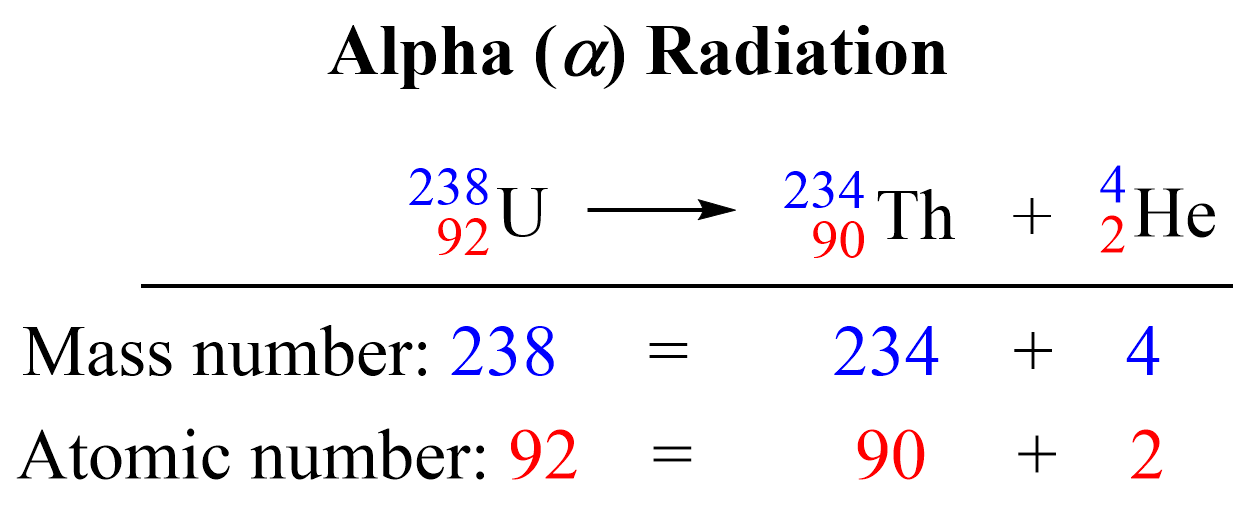
Alpha decay involves the emission of an alpha particle, which is essentially a helium nucleus comprising two protons and two neutrons. Here’s how you can identify an alpha decay reaction:
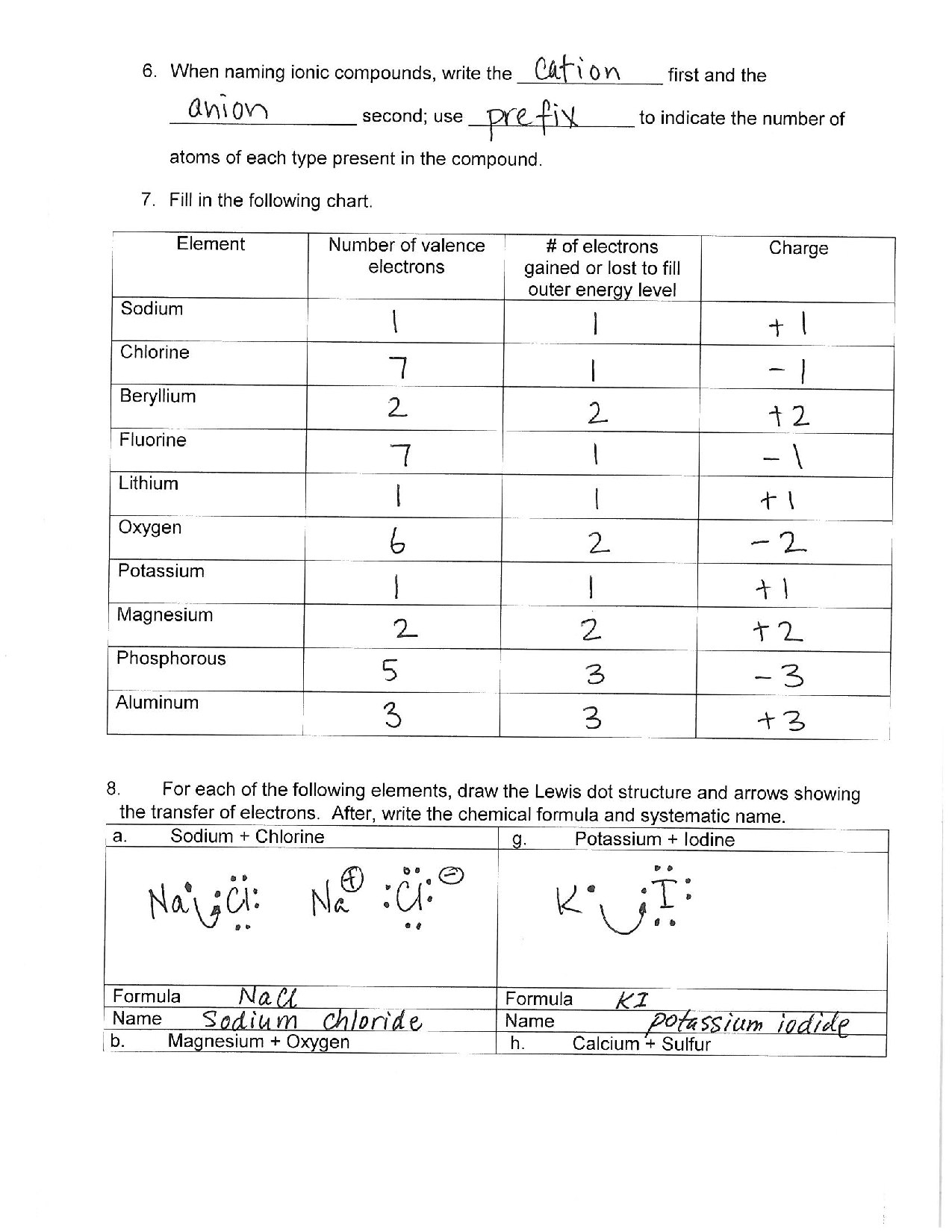
- The daughter nucleus will have an atomic number reduced by 2 and a mass number reduced by 4.
- Alpha particles are relatively heavy and are less penetrating due to their size.
Let's explore this through a worksheet example:
| Parent Nucleus | Daughter Nucleus | Alpha Particle Emitted |
|---|---|---|
| 238U | 234Th | 4He |

💡 Note: In alpha decay, the atomic number decreases by 2 because an alpha particle contains 2 protons.
Beta Decay: Understanding Electron Emission

Beta decay occurs in two forms: beta-minus and beta-plus. Here’s a breakdown:
- Beta-minus decay (β−): A neutron inside the nucleus converts into a proton, an electron (beta particle), and an antineutrino.
- Beta-plus decay (β+): A proton inside the nucleus converts into a neutron, a positron, and a neutrino.
Worksheet example for beta-minus decay:
| Parent Nucleus | Daughter Nucleus | Beta Particle | Antineutrino |
|---|---|---|---|
| 14C | 14N | β− | āν |
📌 Note: Unlike alpha decay, beta decay does not significantly change the mass number of the atom because the mass of a beta particle is negligible.
Worksheet: Mastering Alpha and Beta Decay

Now, let’s put your knowledge into practice with a detailed worksheet:
Problem 1: Alpha Decay

Question: 226Ra undergoes alpha decay. What will be the resulting nucleus and what particle is emitted?
- Solution: The daughter nucleus will be 222Ra, and an alpha particle (4He) is emitted.
Problem 2: Beta Decay

Question: 60Co undergoes beta-minus decay. Identify the daughter nucleus and the particles emitted.
- Solution: The daughter nucleus is 60Ni, a beta particle (β−) and an antineutrino (āν) are emitted.
Problem 3: Sequential Decay

Question: 238U decays through a series of alpha and beta decays to reach 206Pb. Summarize this decay process.
- Solution: Here, 8 alpha decays and 6 beta decays are required to convert Uranium-238 into Lead-206. This process can be summarized as follows:
- 8 * 4He → 8 alpha particles
- 6 * β− → 6 beta particles
- Atomic number decreases by (2*8 - 6) = 10
- Mass number decreases by (4*8 - 0) = 32
⚠️ Note: In real-life decay series, there can be various intermediate isotopes before reaching the final stable isotope.
To sum up our exploration, mastering alpha and beta decay requires an understanding of how atomic nuclei change their structure to achieve stability. Through this worksheet, we've covered not only the theoretical aspects but also practical examples, demonstrating how these decays occur and affect the elements involved. This knowledge not only prepares you for exams or assignments but also provides insight into the fundamental processes that shape our universe.
What causes an atom to undergo alpha or beta decay?

+
The nucleus of an unstable atom naturally seeks stability. Alpha decay occurs when the nucleus has too many protons which are not bound strongly due to electrostatic repulsion, thus emitting an alpha particle reduces the instability. Beta decay happens when there’s an imbalance between protons and neutrons; either a neutron turns into a proton (beta-minus) or a proton turns into a neutron (beta-plus) to achieve equilibrium.
How can we differentiate between alpha and beta particles?

+
Alpha particles are essentially helium nuclei with two protons and two neutrons, making them relatively heavy, positively charged, and less penetrating. Beta particles, on the other hand, are fast-moving electrons (β−) or positrons (β+), which are much lighter and can penetrate more than alpha particles due to their lower mass and charge.
Why is understanding alpha and beta decay important?

+
Understanding alpha and beta decay is essential in various fields. In medicine, it’s used for treatments like radiation therapy. In dating methods like carbon dating, beta decay helps determine the age of organic materials. In physics and chemistry, it’s foundational to nuclear reactions, providing insights into the behavior of atoms and their constituents, which are crucial for nuclear power, astrophysics, and elemental transmutations.