Ionic Formulas Worksheet Answer Key: 5 Essential Tips

Embarking on the journey of mastering chemistry often involves a deep dive into the intricate world of compounds and their compositions. One pivotal area of study within this realm is ionic formulas, where understanding the correct construction and balance of chemical equations is paramount. This comprehensive guide will serve as an answer key and provide you with 5 essential tips on how to effectively use an ionic formulas worksheet, ensuring you grasp the basics, avoid common pitfalls, and enhance your chemical writing skills. Whether you're a student, an educator, or simply someone with a passion for chemistry, this post will elucidate how to conquer the worksheet and understand the science behind ionic compounds.
Understanding Ionic Compounds

Before we delve into the worksheet and our strategies for tackling it, let's establish a foundation in the concepts of ionic compounds:
- Definition: Ionic compounds are formed when a metal donates one or more electrons to a non-metal, resulting in oppositely charged ions that attract each other.
- Charge Balance: The formula of an ionic compound reflects the balance of charges to achieve neutrality. For instance, the formula for sodium chloride is NaCl, where sodium (Na+) and chloride (Cl-) have charges that cancel each other out.
Understanding the concept of charge and how elements strive for stability by gaining or losing electrons is crucial for writing accurate ionic formulas.

Tip 1: Review The Periodic Table

The periodic table is an indispensable tool when dealing with ionic formulas. Here are the key points:
- Learn the common valences (charges) of elements, especially the alkali metals, alkaline earth metals, halogens, and noble gases.
- Understand the trends in the periodic table, such as electronegativity, which indicates an element's affinity for electrons.
- Remember that transition metals can have multiple charges, so they often require a Roman numeral in their names to indicate the charge (e.g., Fe(II) or Fe(III)).
A good grasp of these fundamentals will reduce errors when writing ionic formulas.
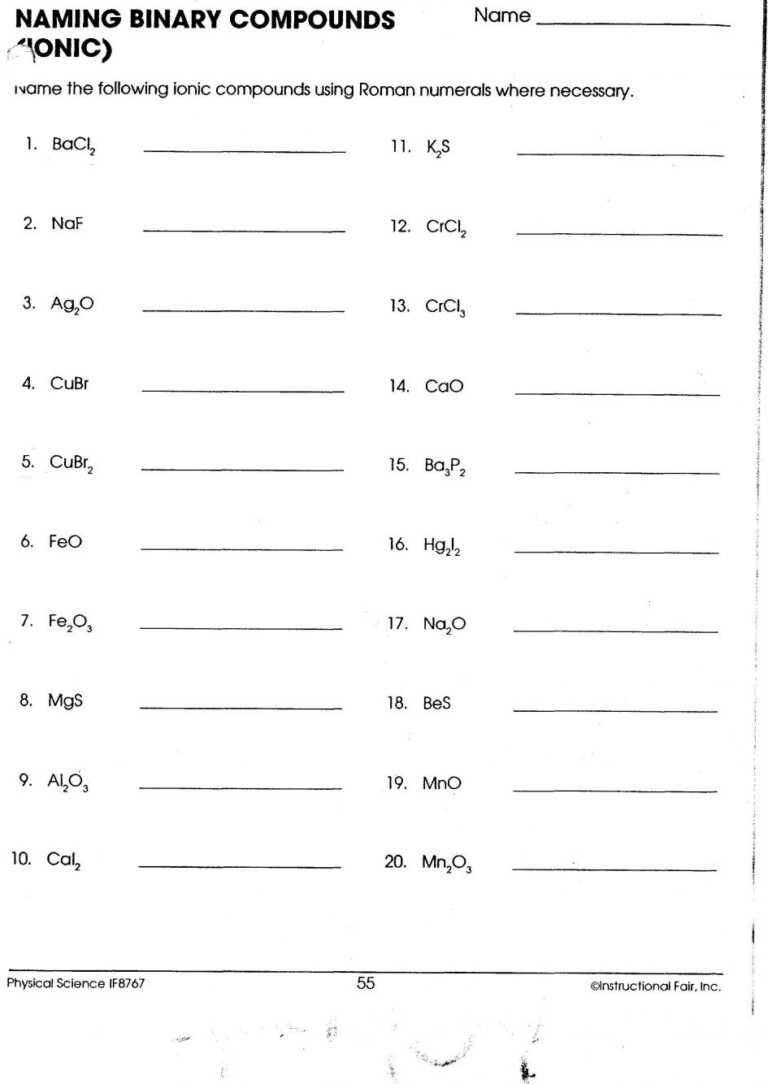
Tip 2: Recognize Common Polyatomic Ions
Polyatomic ions are groups of atoms that carry a net charge. Here's how to approach them:
- Familiarize yourself with the common polyatomic ions such as nitrate (NO3-), sulfate (SO42-), and ammonium (NH4+).
- Memorize their charges and structural formulas for quick reference.
- Practice writing formulas that involve these ions, as they often appear in more complex ionic compounds.
Mastering polyatomic ions can significantly streamline your work when you come across them in a worksheet.
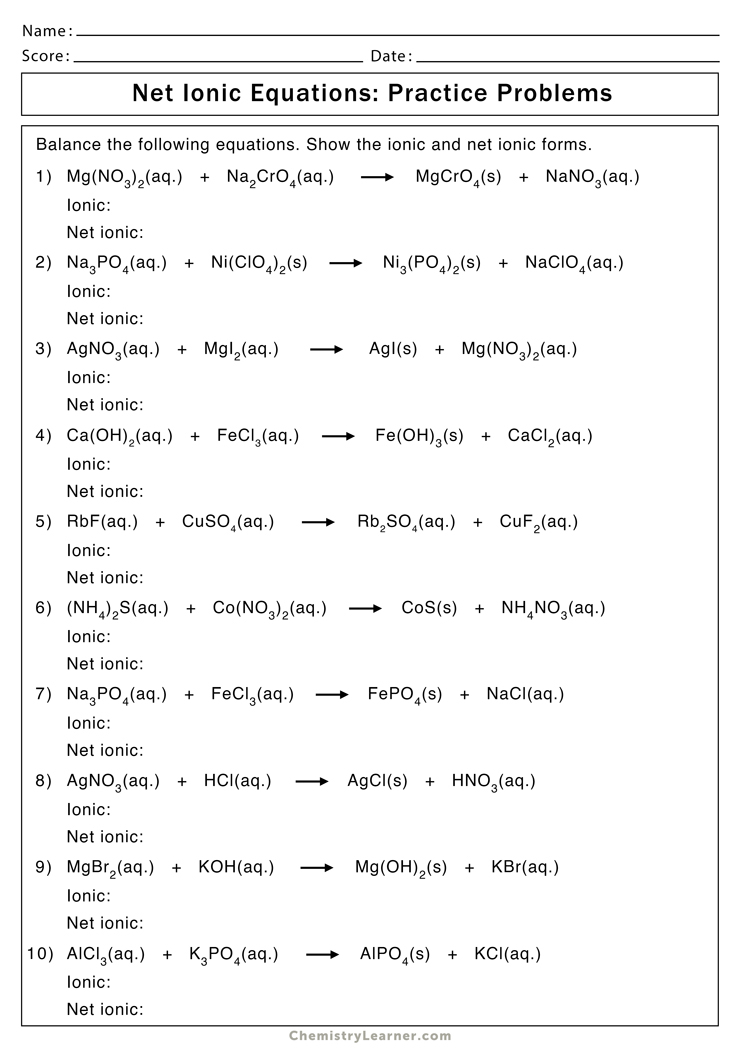
Tip 3: Charge Balancing Is Key

Creating correct ionic formulas necessitates ensuring that the sum of charges from the cation(s) and anion(s) equals zero. Here are some tips:
- Identify the charge of each ion first.
- Use the "crossing-over" method where the charge of one ion becomes the subscript of the other to balance charges.
- Remember that when the charges are the same, you don't need subscripts (e.g., CaCl2 or MgF2).
By keeping an eye on the balance of charges, you can avoid mistakes in your formulas.
| Element | Charge | Example Formula |
|---|---|---|
| Sodium (Na) | +1 | NaCl |
| Calcium (Ca) | +2 | CaCO3 |
| Aluminum (Al) | +3 | Al2(SO4)3 |

⚗️ Note: Always double-check the subscripts in your formulas to ensure they are correctly balanced.
Tip 4: Use Mnemonics and Tricks

Learning can be fun when you make use of mnemonics:
- Create memory aids for remembering charges of common ions (e.g., "Killer Karl, the Clean Counter," to remember that potassium (K) is +1 and chlorine (Cl) is -1).
- Use tricks like the "charge check" to ensure you've balanced correctly (e.g., the positive charges should equal the negative charges).
Mnemonics and tricks can simplify complex rules into manageable, memorable bits.
Tip 5: Practice and Review

Lastly, proficiency in writing ionic formulas comes from:
- Repeated practice using worksheets or digital resources to solidify your knowledge.
- Reviewing and correcting your own work, identifying common mistakes, and learning from them.
- Collaborating with peers to discuss solutions and different approaches to the same problem.
Regular practice not only improves your skill but also boosts your confidence in dealing with any chemical formula challenge.
Mastering ionic formulas is a cornerstone of chemical education. This guide has outlined five essential tips to help you navigate the complexities of ionic formulas worksheets. By understanding the basics, recognizing common polyatomic ions, balancing charges, using mnemonic devices, and practicing regularly, you can become adept in this fundamental aspect of chemistry. Remember, each compound tells a story of electron exchanges, stability, and the creation of new substances, making your journey through chemistry both enlightening and rewarding.
What is an ionic compound?
+
An ionic compound is formed when a metal (cation) donates electrons to a non-metal (anion), creating oppositely charged ions that attract each other to form a stable compound.
Why is balancing charge important in ionic formulas?

+
Balancing charge ensures that the compound is electrically neutral, which is necessary for its stability and to accurately represent the real-world interaction between ions.
How can mnemonics help in learning ionic formulas?

+
Mnemonics provide a memory aid for remembering the charges and formulas of common ions, making it easier to recall information during problem-solving.