5 Steps to Mastering Half Reactions Easily

Understanding half reactions is crucial for anyone studying chemistry, particularly when delving into redox reactions, which are fundamental in electrochemical cells, corrosion studies, and industrial processes. In this post, we'll explore five straightforward steps to make learning and applying half reactions more manageable.
Step 1: Grasping the Concept of Oxidation and Reduction
The journey to mastering half reactions begins with understanding the core concepts:
- Oxidation: This is the process where a species loses electrons. You can remember this by thinking of “OIL” (Oxidation Is Loss of electrons).
- Reduction: Conversely, reduction involves the gain of electrons, which can be recalled using “RIG” (Reduction Is Gain of electrons).
🔍 Note: Keep in mind that oxidation and reduction always occur simultaneously, forming a redox reaction.
Step 2: Identifying the Reacting Species

Once you understand oxidation and reduction, identify the species involved:
- Reducing Agent: The species that gets oxidized. This agent loses electrons, thereby reducing another species.
- Oxidizing Agent: The species that gets reduced. It gains electrons from the reducing agent, which leads to its reduction.
It’s useful to create a table to compare these agents:
| Agent | Role in Reaction | Electrons |
|---|---|---|
| Reducing Agent | Loses electrons | Oxidized |
| Oxidizing Agent | Gains electrons | Reduced |
Step 3: Writing the Half-Reactions
After identifying the agents, write the respective half-reactions:
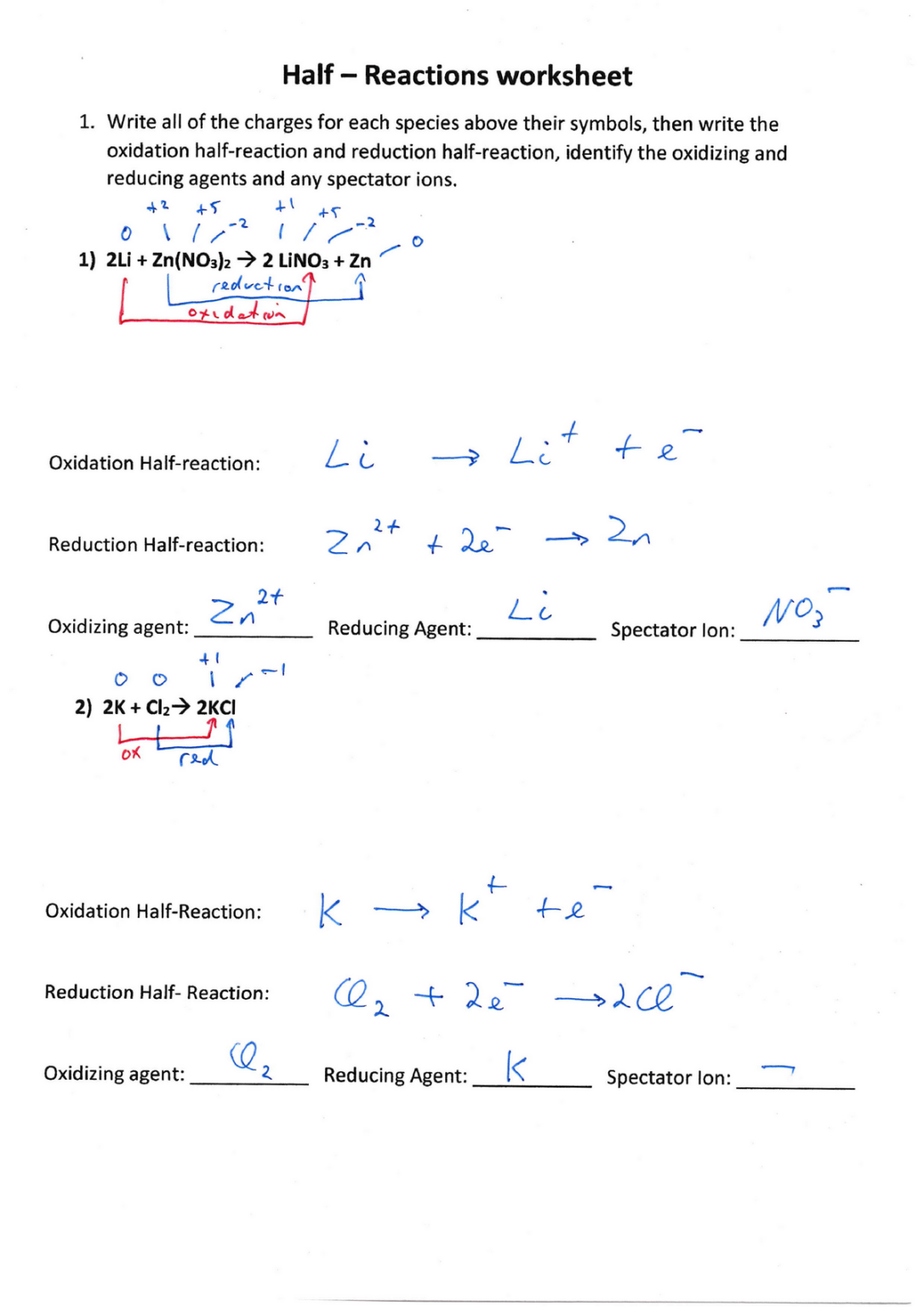
- Oxidation Half-Reaction: Write the equation where the reducing agent loses electrons. For example, in the reaction of zinc with hydrochloric acid, zinc loses electrons: Zn → Zn2+ + 2e-
- Reduction Half-Reaction: Write the equation where the oxidizing agent gains electrons. Continuing with the example, hydrogen ions (from the acid) gain electrons: 2H+ + 2e- → H2
Step 4: Balancing the Half-Reactions

Balancing these half-reactions involves:
- Charge Balance: Ensure that the charge on both sides of the equation is balanced by adjusting the number of electrons involved.
- Atom Balance: Add water molecules (H2O) and hydrogen ions (H+) in acidic solutions or hydroxyl ions (OH-) in basic solutions to balance the number of atoms.
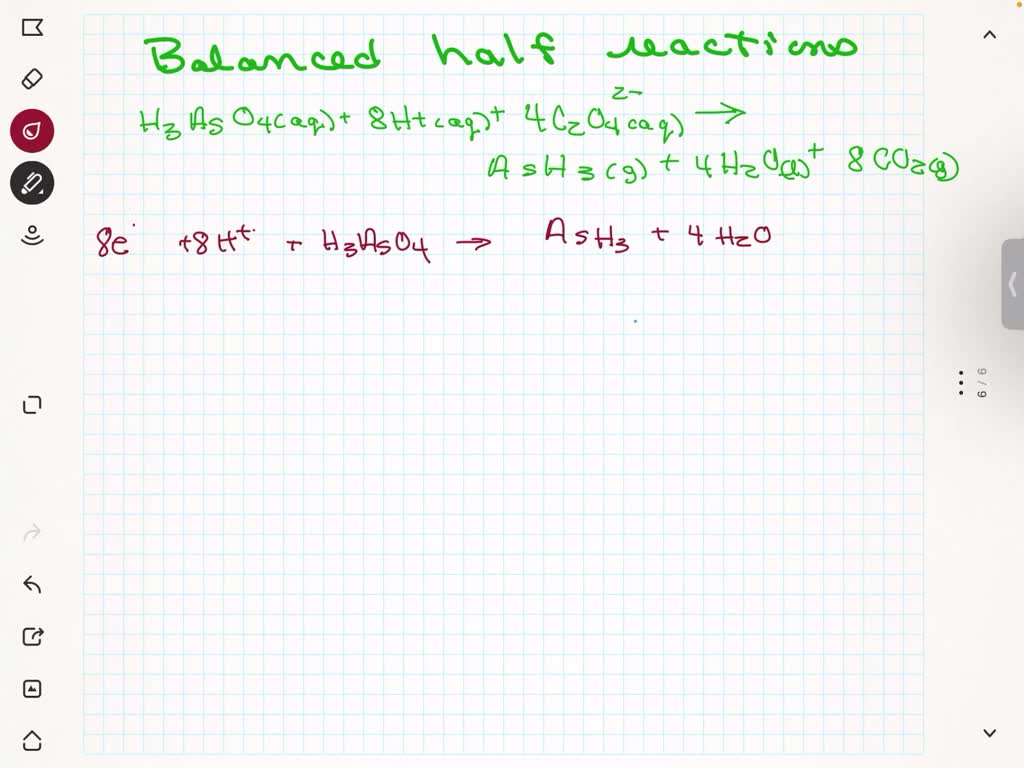
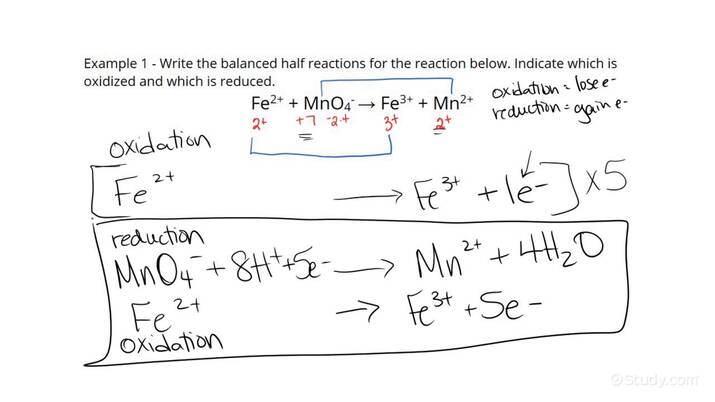
Here’s an example for the reaction between permanganate ion (MnO4-) and iron (II) (Fe2+):
Oxidation: 5 Fe2+ → 5 Fe3+ + 5 e-Reduction: MnO4- + 5e- + 8H+ → Mn2+ + 4H2O
Step 5: Combining the Half-Reactions

To get the net ionic equation:
- Cancel Electrons: Ensure the number of electrons lost in oxidation equals the number gained in reduction by multiplying if necessary.
- Add the Equations: Combine the balanced half-reactions by canceling out common terms (like electrons) on both sides.
For the reaction between Fe2+ and MnO4-, the complete reaction would be:
5Fe2+ + MnO4- + 8H+ → 5Fe3+ + Mn2+ + 4H2O
Throughout these steps, maintaining accuracy in balancing the half-reactions ensures that the overall redox reaction is correctly represented. Understanding half reactions not only aids in predicting the outcome of chemical reactions but also enhances your ability to manipulate chemical processes for desired outcomes.
Why do we need to balance half reactions?

+
Balancing half reactions ensures that both mass and charge are conserved in the overall redox equation. This balance reflects the stoichiometry of the reaction, ensuring that no electrons, atoms, or charges are created or destroyed, which is a fundamental principle in chemistry.
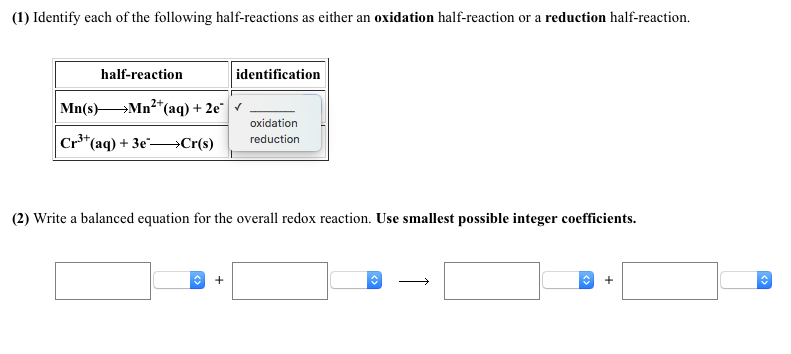
How do you know if a half reaction is oxidation or reduction?

+
If a species loses electrons, it’s undergoing oxidation. If it gains electrons, it’s experiencing reduction. Remember, “OIL” and “RIG” - Oxidation Is Loss, Reduction Is Gain.
Can redox reactions occur without oxygen?

+
Yes, redox reactions do not require oxygen. “Redox” stands for reduction-oxidation, and these processes involve the transfer of electrons between species, regardless of whether oxygen is involved.
What are some common errors in balancing half reactions?

+
Common errors include not balancing the charges, forgetting to add water or hydrogen ions, and not ensuring that the number of electrons lost equals the number gained. Also, overlooking the reaction medium (acidic or basic) can lead to mistakes in balancing.
How does understanding half reactions help in practical applications?
+
Understanding half reactions is key in industries like battery design, electroplating, wastewater treatment, and corrosion control. It helps in predicting and controlling the direction of redox reactions, thereby optimizing processes or preventing unwanted reactions.



