5 Tips for Mastering Ionic Compound Formulas

Understanding Ionic Bonds: The Basics

Ionic compounds are formed when atoms exchange electrons to achieve a stable octet configuration. Understanding the basics of ionic bonding is essential for mastering the formulas and properties of ionic compounds. Here’s a breakdown of the key concepts:
- Electronegativity: The atom that has the higher electronegativity will attract electrons more strongly, thus gaining electrons to become negatively charged (an anion).
- Charge Balance: For an ionic compound to form, the sum of the charges of the cation(s) and anion(s) must be zero. This means that the total positive charge equals the total negative charge.
- Periodic Table: Elements’ positions on the periodic table can predict their ionic behavior; alkali metals (Group 1) form +1 ions, while halogens (Group 17) form -1 ions.
💡 Note: When using the octet rule, consider exceptions such as elements in higher periods that might achieve stability beyond eight electrons.
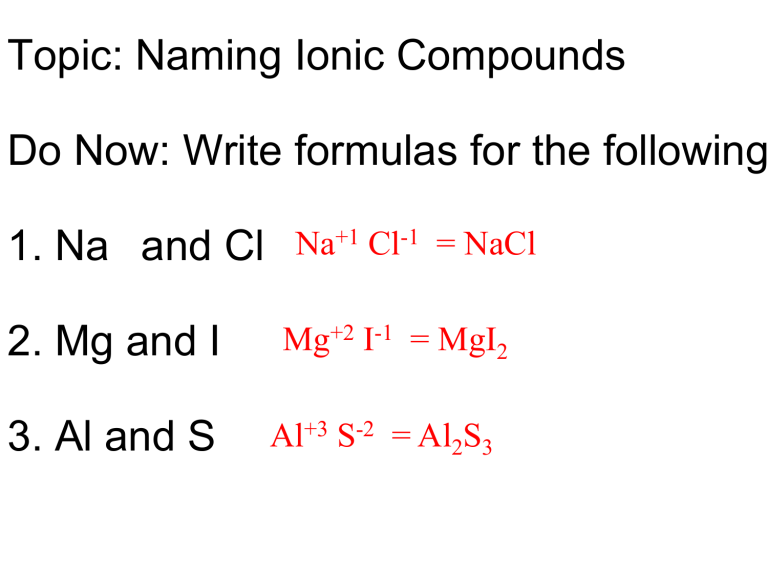
Tip 1: Use The Cross-Over Method

The cross-over method is a straightforward technique to find the simplest formula of an ionic compound:
- Write down the charges of each ion involved.
- Cross over the charges and make them the subscripts of the opposite ion.
- Reduce the subscripts to the simplest ratio, if necessary.
| Cation | Anion | Formula |
|---|---|---|
| Ca2+ | Cl- | CaCl2 |
| Na+ | S2- | Na2S |
Tip 2: Know Your Polyatomic Ions

Polyatomic ions are charged entities made up of two or more atoms. Here are some common polyatomic ions:
- Nitrate: NO3-
- Sulfate: SO42-
- Phosphate: PO43-
- Ammonium: NH4+
When working with polyatomic ions, remember to use parentheses when the ion's subscript changes due to balancing the charge:
- Al(NO3)3 instead of AlNO33
Tip 3: Practice with Examples

The best way to learn ionic compound formulas is by practicing with various examples. Here are some examples for practice:
- Magnesium oxide (MgO): Mg2+ + O2-
- Aluminum fluoride (AlF3): Al3+ + 3F-
- Calcium carbonate (CaCO3): Ca2+ + CO32-
🔍 Note: Balancing the charges correctly is key, and understanding exceptions to common valence states can be crucial.
Tip 4: Learn Common Valence States

Recognizing common valence states can streamline the process of writing formulas:
- Aluminum: Al3+
- Calcium: Ca2+
- Sodium: Na+
However, some elements can exhibit more than one valence state:
- Iron: Fe2+ or Fe3+
- Copper: Cu+ or Cu2+
Tip 5: Consider the Naming Rules

Naming ionic compounds is linked to their formulas:
- The cation is written first and named as is.
- The anion changes its ending to ‘-ide’ or ‘-ate’ for polyatomic ions.
- If the metal has multiple common charges, the charge of the metal is specified with Roman numerals.
Take the example of Iron(II) chloride (FeCl2) or Iron(III) chloride (FeCl3). The name indicates the charge on the iron ion.
📝 Note: Learning the rules for naming ionic compounds can help predict the chemical composition from the name and vice versa.
In summary, mastering ionic compound formulas involves understanding ionic bonds, using the cross-over method, learning polyatomic ions, practicing with examples, knowing common valence states, and being adept with naming rules. These tips not only help you write correct formulas but also facilitate your understanding of the chemical world around you, leading to better comprehension of how substances interact and form bonds. This knowledge is fundamental for anyone studying chemistry, from high school students to professionals in the field.
What is the difference between an ionic and covalent bond?

+
Ionic bonds involve the transfer of electrons from one atom to another, typically between a metal and a non-metal, leading to a positively charged cation and a negatively charged anion. Covalent bonds, on the other hand, involve the sharing of electrons between two atoms, usually between non-metals, to form molecules.
How do I memorize common polyatomic ions?

+
Flashcards, mnemonics, and regular practice with writing and naming compounds involving polyatomic ions can help. It’s also beneficial to understand the logic behind their formation, like the common -ate and -ite endings.
Can ionic compounds exist as gases?

+
At room temperature, ionic compounds are typically solids with high melting and boiling points. However, they can exist as gases at high temperatures or under conditions where the ionic bond is broken, like in a plasma state.