5 Tips for Writing Ionic Compound Formulas Easily
Understanding the formulas of ionic compounds is a fundamental aspect of chemistry, especially for students in their journey through the sciences. At first glance, the task of writing ionic compound formulas can seem daunting due to the need to grasp the underlying principles of ion formation, charges, and balancing. However, by following a structured approach, this task becomes far simpler. In this post, we will explore five essential tips to make writing ionic compound formulas an easy and understandable process.
Tip 1: Understand Ion Charges
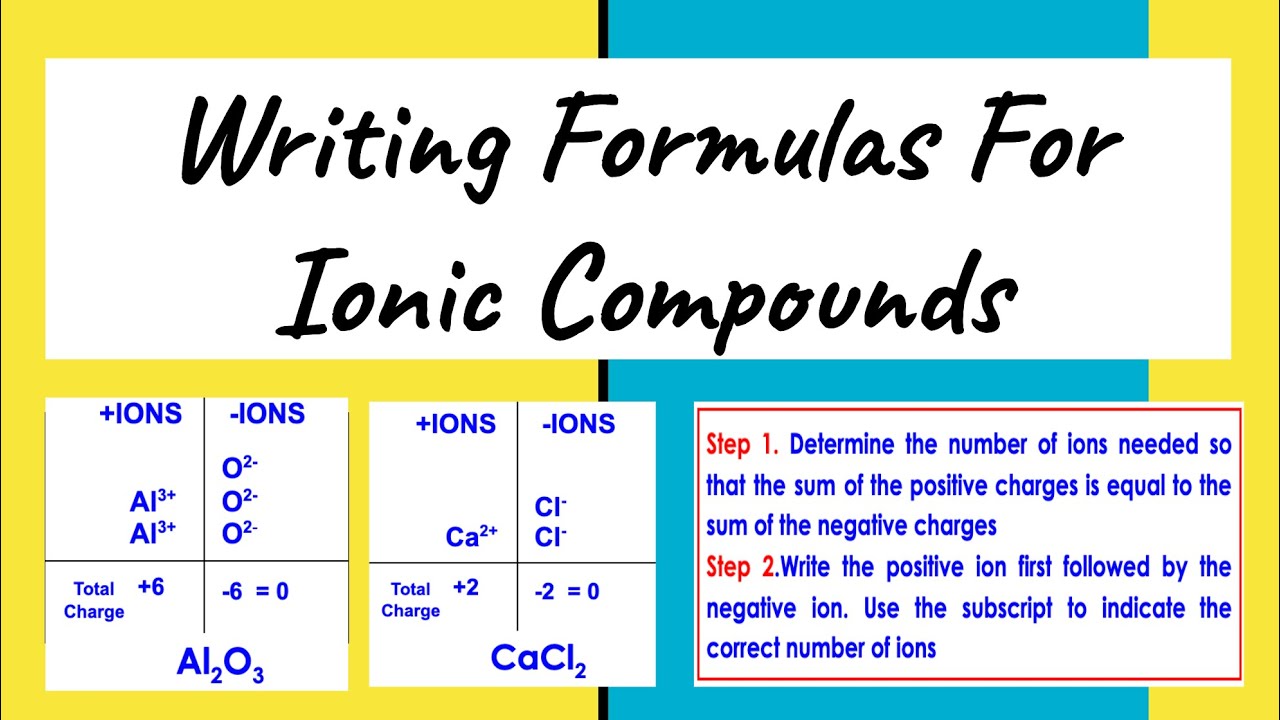
Before you can write the formula of an ionic compound, you need to understand the charge each ion typically carries. Here are some key points:
- Positive Ions (Cations): Metal atoms lose electrons to become cations, gaining a positive charge. The most common metals include sodium (Na+), calcium (Ca2+), and aluminum (Al3+).
- Negative Ions (Anions): Non-metal atoms gain electrons to form anions, acquiring a negative charge. For instance, chlorine (Cl-), oxygen (O2-), and nitrogen (N3-) are frequently encountered anions.
Knowing these common charges is the first step in correctly determining formulas.
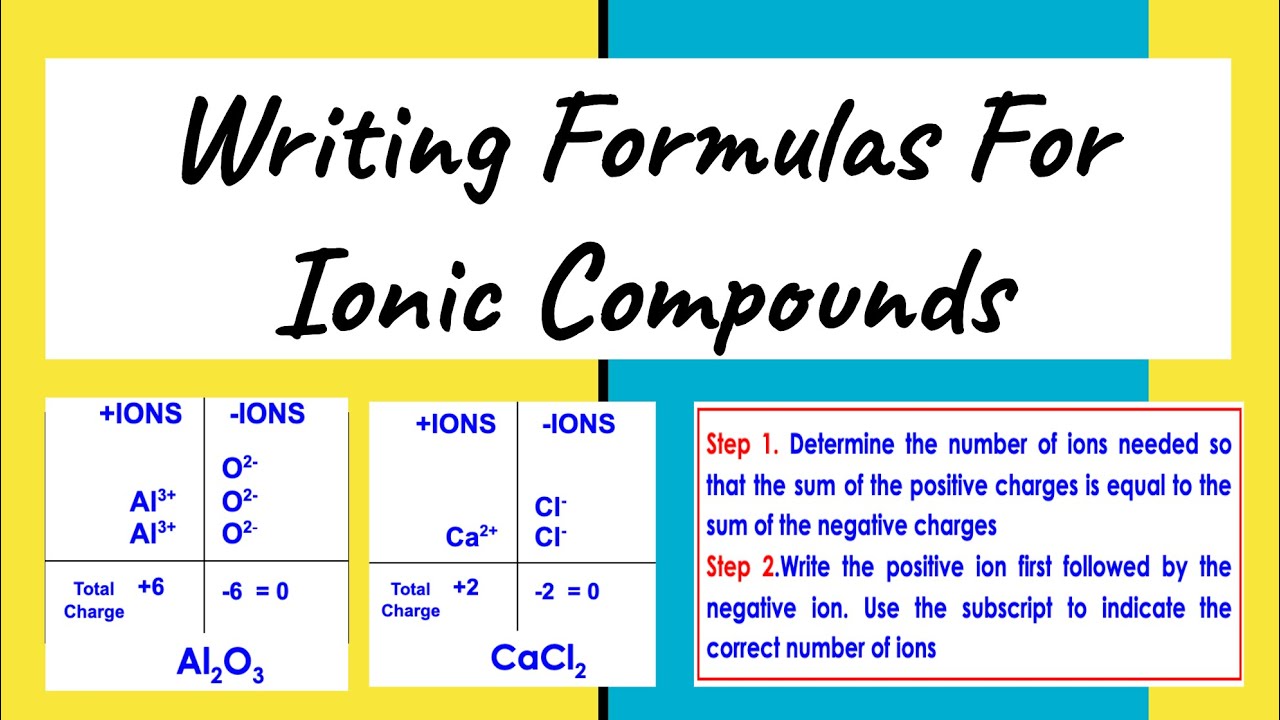
Tip 2: Balance Charges with the Crisscross Method
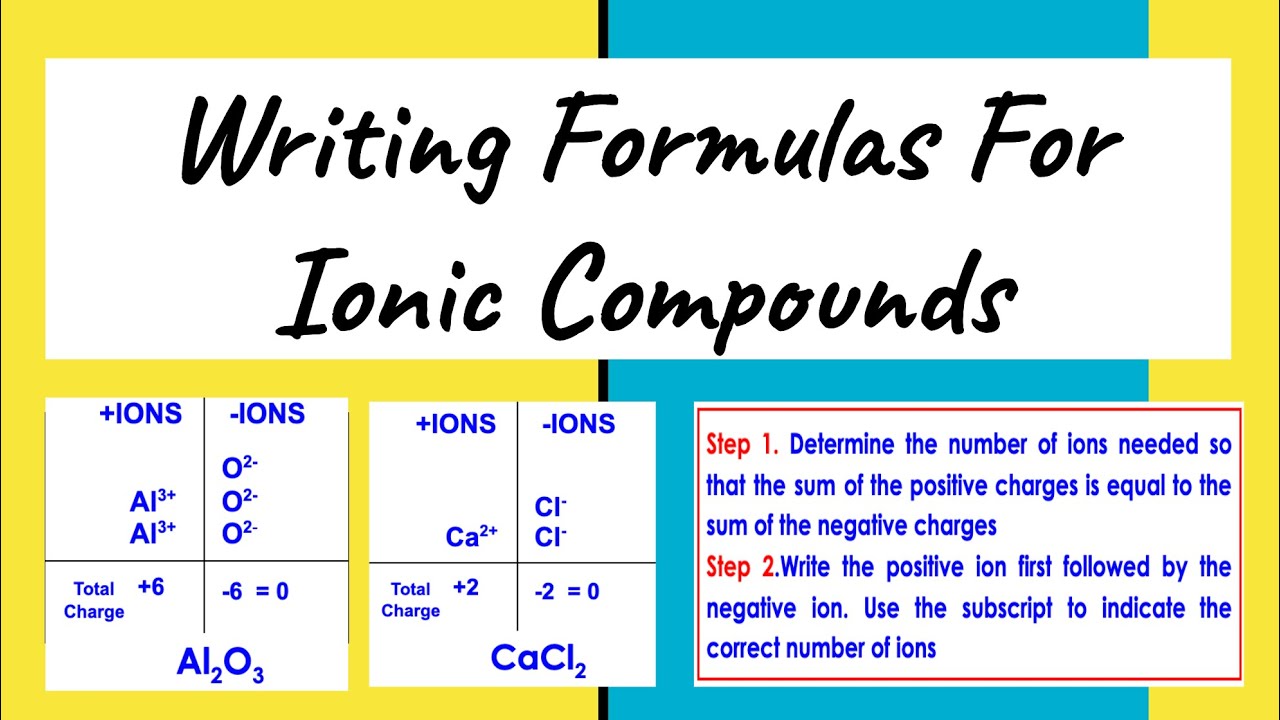
One of the most straightforward methods to write an ionic formula is the crisscross method:
- Write the symbol of the cation first followed by the anion.
- Crisscross the charge numbers, making them subscripts to the opposite ion to balance charges.
Here is an example with sodium and chlorine to form sodium chloride:
| Steps | Example |
|---|---|
| Write Symbols | Na Cl |
| Crisscross Charges | Na1Cl1 (Charge of sodium = +1, chlorine = -1) |
| Reduce subscripts to lowest terms | NaCl (result) |
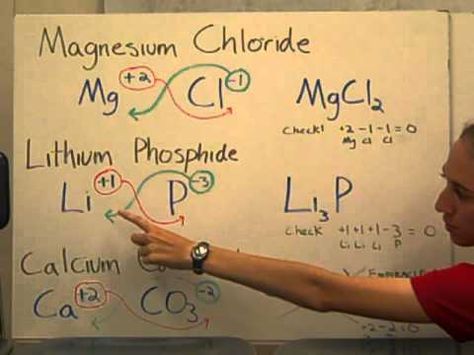
Remember, if the charges are the same, the subscripts will be the same and vice versa.
Tip 3: Consider Polyatomic Ions
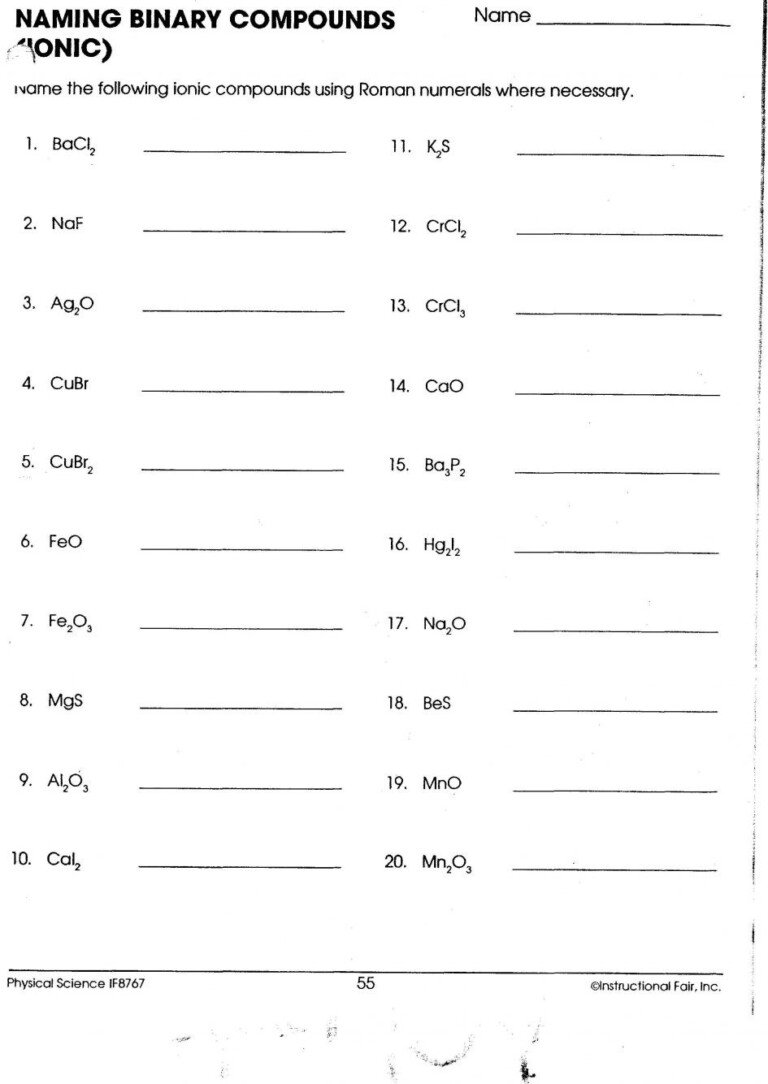
Polyatomic ions are ions composed of two or more atoms that carry a charge. Examples include:
- Sulfate (SO42-)
- Ammonium (NH4+)
- Nitrate (NO3-)
When dealing with these, follow these guidelines:
- Use parentheses if you need multiple units of a polyatomic ion to balance the charges.
- Remember to balance the overall charge, as with simple ions.
Tip 4: Always Check Your Work

It’s crucial to double-check your work by ensuring the net charge of the compound is zero. This is the hallmark of a correct ionic formula:
- Sum the positive and negative charges to ensure they equal zero.
- If they do not, reevaluate your formula to see where adjustments are needed.
Tip 5: Practice with Common Compounds
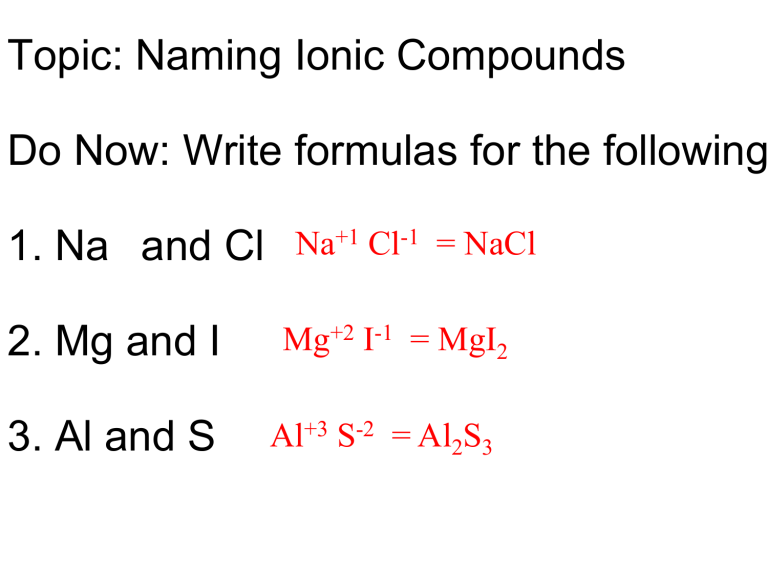
Mastery comes with practice. Here are some common compounds to practice with:
- Sodium chloride (NaCl)
- Calcium carbonate (CaCO3)
- Magnesium oxide (MgO)
- Ammonium nitrate (NH4NO3)
Regularly practicing these will help reinforce your understanding of ionic compound formulas.
As you familiarize yourself with these tips, writing ionic compound formulas will transition from a complex task to an intuitive one. Learning to recognize common ions, understanding the balance of charges, and consistently checking your work are key. Practice with various compounds, including both simple and polyatomic ions, will cement your ability to write formulas effortlessly.
✍️ Note: Always keep in mind that while the methods outlined here work for most ionic compounds, there are exceptions in chemistry, particularly with transition metals where variable charges need consideration.
In essence, mastering the art of writing ionic compound formulas involves understanding ion charges, balancing these charges, recognizing and incorporating polyatomic ions, and practicing these principles. By following these steps, your approach to chemistry will not only become more methodical but also more insightful, leading to a deeper appreciation of the subject's underlying principles.
What is an ionic compound?

+
An ionic compound is a chemical compound composed of ions held together by electrostatic forces termed ionic bonding. The ions form by atoms losing or gaining electrons to achieve a stable electron configuration, typically that of noble gases.
Can an ion have more than one charge?
+
Yes, especially with transition metals, which can exhibit multiple oxidation states. For example, iron can be Fe2+ or Fe3+. When these are present, the charge must be specified in the ion’s name or formula.
How do you know when to use parentheses in ionic formulas?
+
Use parentheses when a polyatomic ion is repeated in the formula more than once to indicate how many times the group is present. For example, in calcium phosphate, Ca3(PO4)2, the PO4 ion is enclosed in parentheses to indicate there are two units.