Master Compound Naming with This Ultimate Worksheet

Mastering the art of naming chemical compounds is essential for students of chemistry. Not only does it pave the way for understanding more complex chemical concepts, but it also ensures clarity in communication among scientists. Whether you're tackling ionic compounds, covalent compounds, or acids and bases, understanding their nomenclature is foundational. Here’s an ultimate worksheet designed to guide you through the complex maze of chemical naming with tips, rules, and plenty of practice questions.
Basic Principles of Compound Naming


Before diving into specific types of compounds, let’s establish some fundamental principles:
- Systematic Naming: The IUPAC (International Union of Pure and Applied Chemistry) provides a standard method for naming compounds systematically, ensuring global consistency.
- Common Names: Some compounds have historical or common names that might not follow systematic rules but are widely accepted.
- Prefixes, Suffixes, and Roots: Different parts of a chemical name have specific meanings related to the type, number, or state of elements or ions involved.
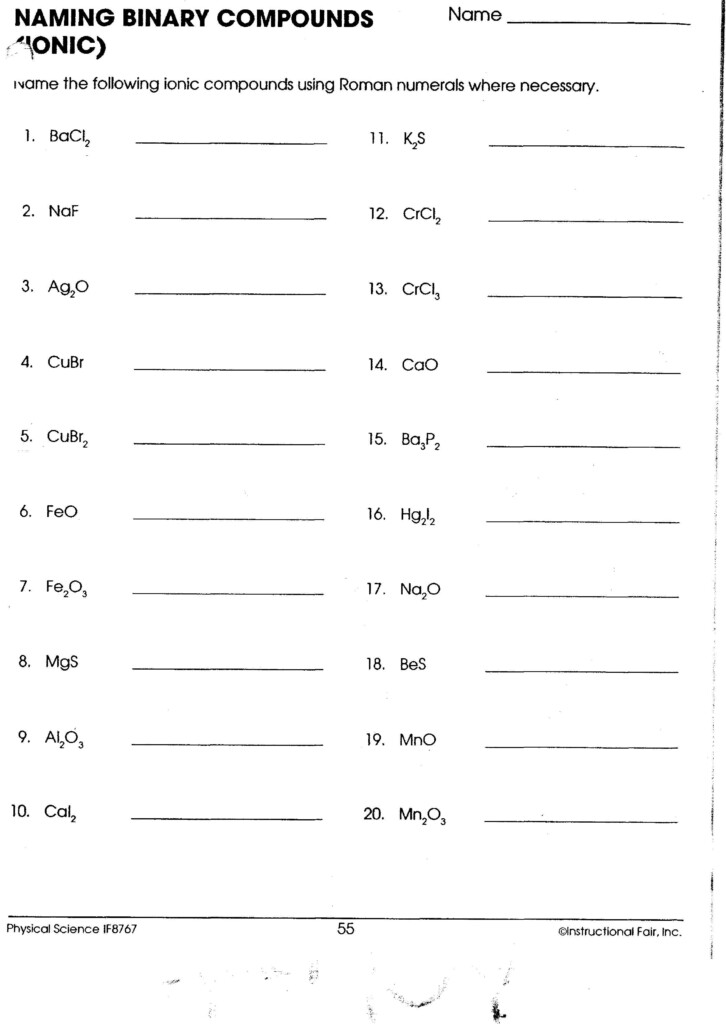
Ionic Compounds

Ionic compounds consist of positively charged ions (cations) and negatively charged ions (anions). Here’s how you can name them:
- Name the cation: If it’s a metal with only one possible charge (e.g., Sodium Na+), simply use the element’s name. For transition metals with variable charges (e.g., Iron), specify the charge with Roman numerals in parentheses.
- Name the anion: Drop the ending of the element name and add “-ide” (e.g., Chlorine -> Chloride).
- Adjust for polyatomic ions: Many polyatomic ions retain their names when part of a compound (e.g., Sulfate SO42-).
⚗️ Note: Transition metals often have multiple charges, making Roman numerals necessary to avoid ambiguity in naming.
Practice with Ionic Compounds

| Formula | Name |
|---|---|
| NaCl | Sodium Chloride |
| FeO | Iron(II) Oxide |
| CuSO4 | Copper(II) Sulfate |

Covalent Compounds
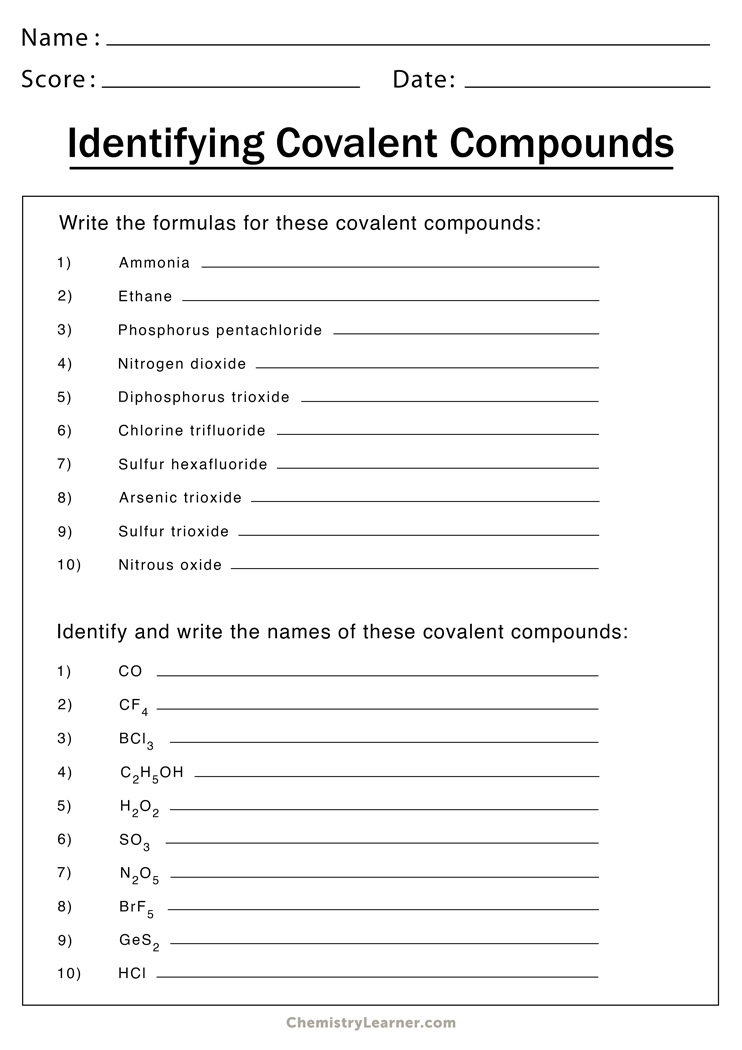
Covalent compounds, or molecular compounds, share electrons between atoms. Naming rules include:
- Use Prefixes: Indicate the number of each atom with prefixes (mono-, di-, tri-, tetra-, etc.).
- Second Element Ending: Always change the ending of the second element to “-ide”.
- Omit Mono- for the First Element: Typically, you do not use “mono” for the first element unless it’s part of the compound’s name.
Practice with Covalent Compounds

- P2O5: Diphosphorus Pentoxide
- CO:
- N2O:
⚗️ Note: When writing names for covalent compounds, remember that prefixes are essential for distinguishing different compounds with the same elements.
Acids and Bases
Naming acids and bases involves a bit of memorization but follows some logical patterns:
Acids:

- Hydro + Non-Oxygen containing: “Hydro” + nonmetal root + “-ic” + “acid” (e.g., HCl: Hydrochloric Acid)
- Oxyacids (contain oxygen):
- Use the root of the nonmetal followed by “-ic” or “-ous” (e.g., HNO3: Nitric Acid)
- The suffix “-ate” in the anion becomes “-ic”, while “-ite” becomes “-ous”.
Bases:

- Essentially, name bases as ionic compounds, with the OH- group as Hydroxide (e.g., NaOH: Sodium Hydroxide).
⚗️ Note: The distinction between "-ic" and "-ous" acids lies in the number of oxygen atoms relative to the anion's base form.
Practice Exercises

Practice is key to mastering compound naming. Here are some exercises:
- Name the following ionic compounds:
- KBr
- AgNO3
- Ca(OH)2
- Provide formulas for the following covalent compounds:
- Carbon Monoxide
- Nitrogen Dioxide
- Silicon Tetrachloride
- Name the following acids:
- H2SO4
- H3PO4
- HF
To wrap up this comprehensive guide on compound naming, remember that while the rules might seem daunting at first, they follow logical patterns. Through practice and understanding the underlying principles, you'll become proficient in chemical nomenclature, which is indispensable for further studies in chemistry. Each compound's name tells a story about its elements, bonding, and properties. As you continue, keep applying these rules, and you'll find the process becoming second nature.
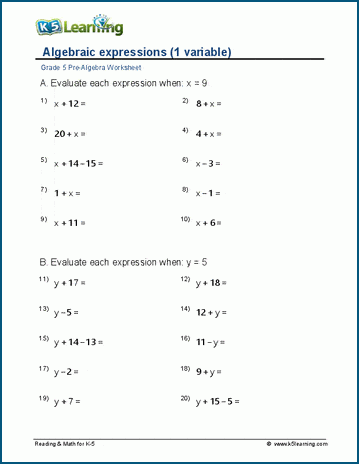
Why do we use Roman numerals when naming some ionic compounds?

+
Roman numerals indicate the charge of transition metal ions in compounds where metals can exist in multiple oxidation states. This helps avoid ambiguity in naming.
What’s the difference between the common name and the systematic name of a compound?

+
A common name might be based on traditional or historical usage, often simpler and more intuitive for everyday use, while a systematic name follows IUPAC rules, ensuring universal understanding and avoiding confusion among scientists.
Can acids have both common and systematic names?
+
Yes, for example, acetic acid has the common name “vinegar” and the systematic name “ethanoic acid” in chemistry contexts.