5 Easy Steps to Master Electron Configuration Worksheets

In the intricate realm of chemistry, understanding the electron configuration of atoms is akin to unlocking a secret code that explains how the elements behave and interact. Electron configurations provide insights into an element's chemical properties, its place in the periodic table, and its bonding capabilities. Mastering electron configuration worksheets is thus a critical skill for students delving into chemistry. This guide breaks down this complex topic into five easy-to-follow steps, enabling learners to navigate electron configuration effortlessly.
Step 1: Understand the Basics of Electron Configuration

Electron configuration is the arrangement of electrons in atomic orbitals around the nucleus of an atom. Here’s what you need to know:
- Electron Shells: Electrons are organized in energy levels called shells. These shells are labeled with numbers starting from 1.
- Subshells: Each shell has subshells (s, p, d, f) that indicate different shapes of orbitals where electrons can reside.
- Aufbau Principle: Electrons occupy orbitals in order of increasing energy, filling lower-energy orbitals first.
- Pauli Exclusion Principle: Each orbital can hold a maximum of two electrons with opposite spins.
- Hund’s Rule: Electrons will singly occupy orbitals of the same energy before pairing up.
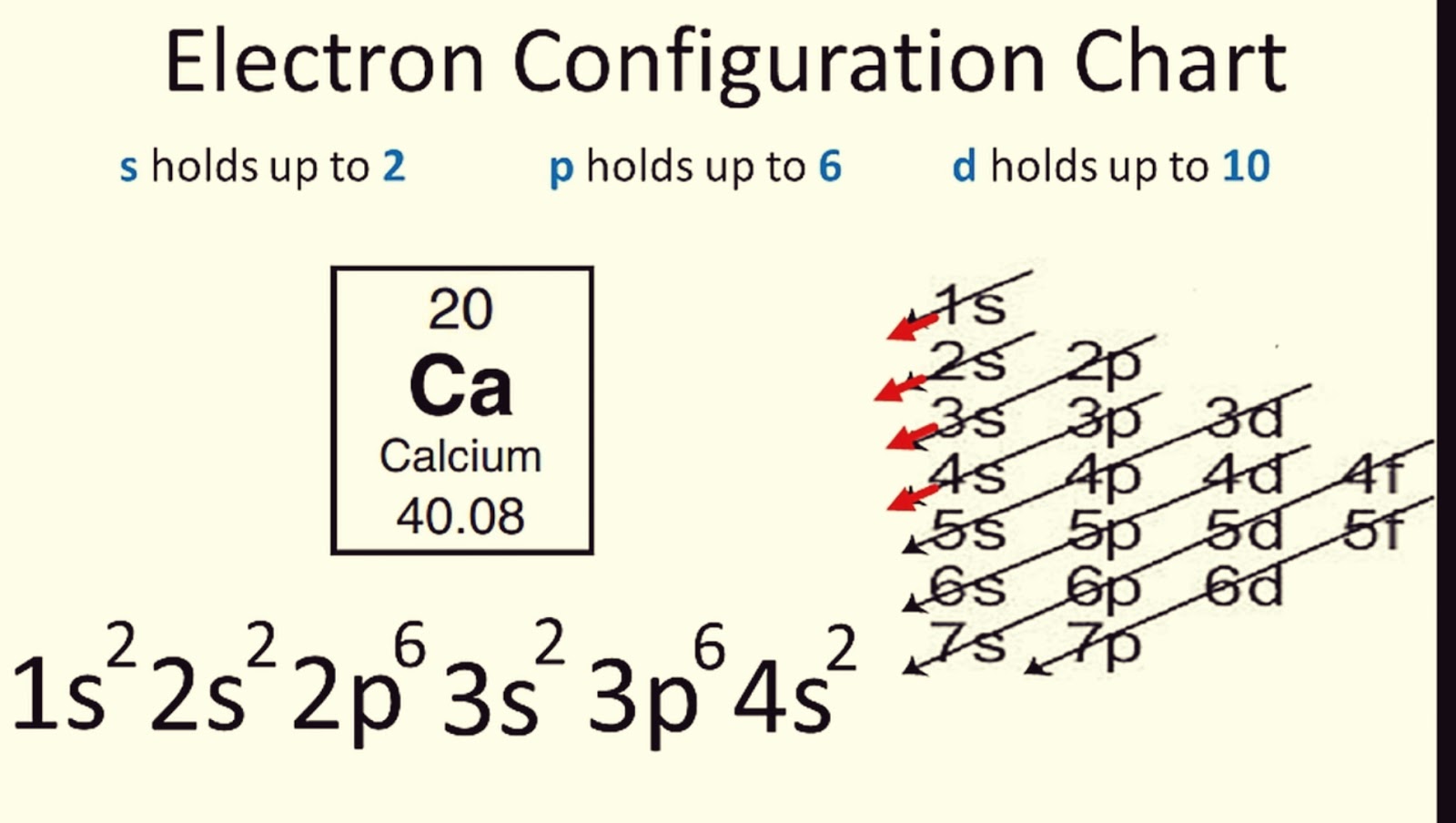
Step 2: Memorize the Periodic Table Structure

The periodic table is a roadmap for electron configurations. Here are key elements to memorize:
- Periods: Rows represent electron shells.
- Blocks: The s, p, d, and f blocks dictate which subshells are being filled.
- Groups: Columns indicate the number of valence electrons in the outermost shell.
💡 Note: Knowing how to use the periodic table to deduce electron configurations can save you time during exams.
Step 3: Utilize Electron Configuration Notation

Electron configuration is written using a shorthand notation:
noble gas core Noble gas symbolHere's how to use this notation:
- Identify the nearest noble gas with a lower atomic number and use its electron configuration as the core.
- List the remaining electrons in the subshells.

| Element | Electron Configuration |
|---|---|
| Sodium (Na) | [Ne]3s1 |
| Magnesium (Mg) | [Ne]3s2 |

Step 4: Practice with Worksheets and Exercises
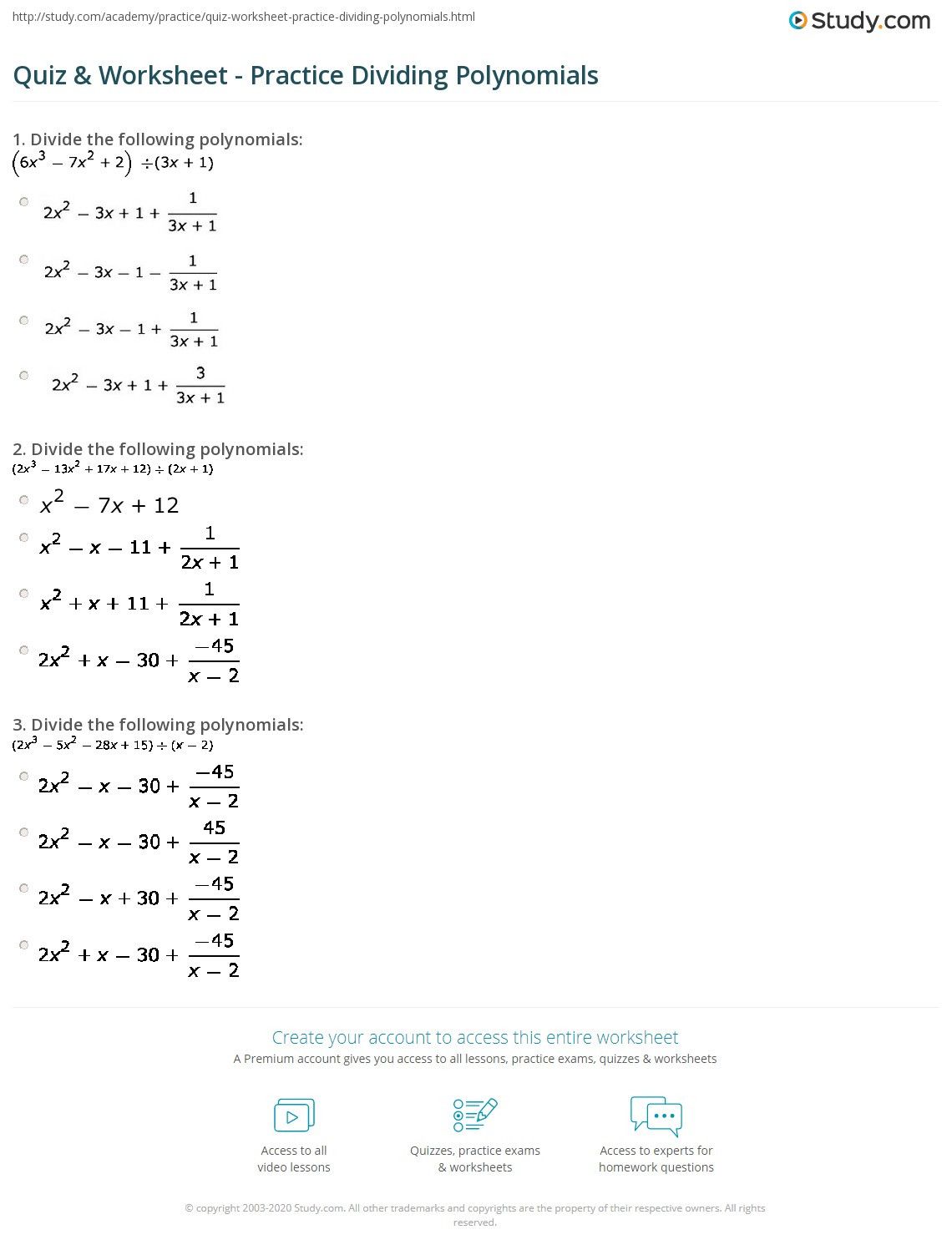
Getting hands-on experience is key:
- Use practice worksheets to identify the electron configurations of various elements.
- Create flashcards to test your memory on the atomic numbers and electron configurations.
- Solve problems that involve electron configurations, such as predicting chemical reactivity or stability.
Step 5: Master Anomalies and Exceptions

While most elements follow the expected electron configurations, some do not:
- Chromium (Cr) and Copper (Cu): These have partially filled d-orbitals, resulting in half-filled or fully-filled subshells for added stability.
- Transition Metals: Look out for exceptions in the d-block elements.
- Lanthanides and Actinides: These series involve filling the f-orbitals, which can be complex.
Understanding these exceptions requires a deeper dive into atomic theory but is crucial for mastering electron configurations.
By following these five steps, you can navigate through electron configuration worksheets with confidence, understanding not just the 'how' but also the 'why' behind these atomic structures. Remember, while electron configurations seem like a rote memorization task, they provide a window into the fascinating world of atomic behavior, chemical reactions, and the beauty of the periodic table.
Why are electron configurations important?

+
Electron configurations help us predict an element’s chemical properties, its place in the periodic table, and how it will interact with other elements. They are essential for understanding chemical reactions, bonding, and electronic structure.
What is the Aufbau principle?

+
The Aufbau principle states that electrons fill orbitals in order of increasing energy. An orbital with lower energy is filled before an orbital with higher energy.
How do I remember the order of electron filling?

+
A helpful mnemonic is to memorize the order as “1s 2s 2p 3s 3p 4s 3d 4p 5s 4d 5p 6s 4f 5d 6p 7s 5f 6d 7p” or use the ‘diagonal’ method where you draw arrows from the bottom to the top in diagonals.