5 Tips for Mastering Chemistry Word Equations Worksheets

Introduction to Chemistry Word Equations

In chemistry, understanding how to translate word descriptions of chemical reactions into balanced equations is an essential skill. Whether you're a high school student or preparing for competitive exams, mastering chemistry word equations can significantly boost your conceptual understanding and problem-solving abilities. This blog post delves into practical tips that can help you excel in tackling chemistry word equations worksheets.
1. Understand the Basics of Chemical Reactions

Before diving into equations, familiarize yourself with the fundamentals:
- Reactants - The starting substances in a chemical reaction.
- Products - The substances produced as a result of the reaction.
- Conservation of Mass - The principle that the total mass of reactants must equal the mass of products.
Knowing these concepts will make it easier to form equations from word descriptions. For example, if you read, "Sodium reacts with chlorine to produce sodium chloride," you'll know:
- Reactants: Sodium (Na) and Chlorine (Cl2)
- Product: Sodium Chloride (NaCl)
💡 Note: Always start with identifying the reactants and products when you translate word problems into equations.
2. Learn Common Chemical Symbols and Formulas

Chemical symbols and formulas are the alphabet of chemistry. Here's a list of some common elements and compounds you'll encounter:
| Element/Compound | Symbol/Formula |
|---|---|
| Hydrogen | H2 |
| Oxygen | O2 |
| Water | H2O |
| Sodium Chloride | NaCl |
| Carbon Dioxide | CO2 |

Familiarity with these will streamline your ability to write equations quickly. For instance, if you see "calcium carbonate reacts with hydrochloric acid," you know:
- Reactants: CaCO3 (calcium carbonate) and HCl (hydrochloric acid)
3. Utilize Keywords to Guide You

Chemistry word equations often use specific keywords that hint at the type of reaction:
- Burns, Reacts, or combines suggest synthesis or combustion.
- Decomposes implies decomposition.
- Replaces indicates single replacement reactions.
By paying attention to these keywords, you can predict the reaction type and anticipate what products might be formed. For example:
- "Magnesium burns in oxygen to form magnesium oxide" would suggest: Mg + O2 → MgO
📝 Note: Recognize these keywords early in your problem-solving process to quickly form a preliminary equation.
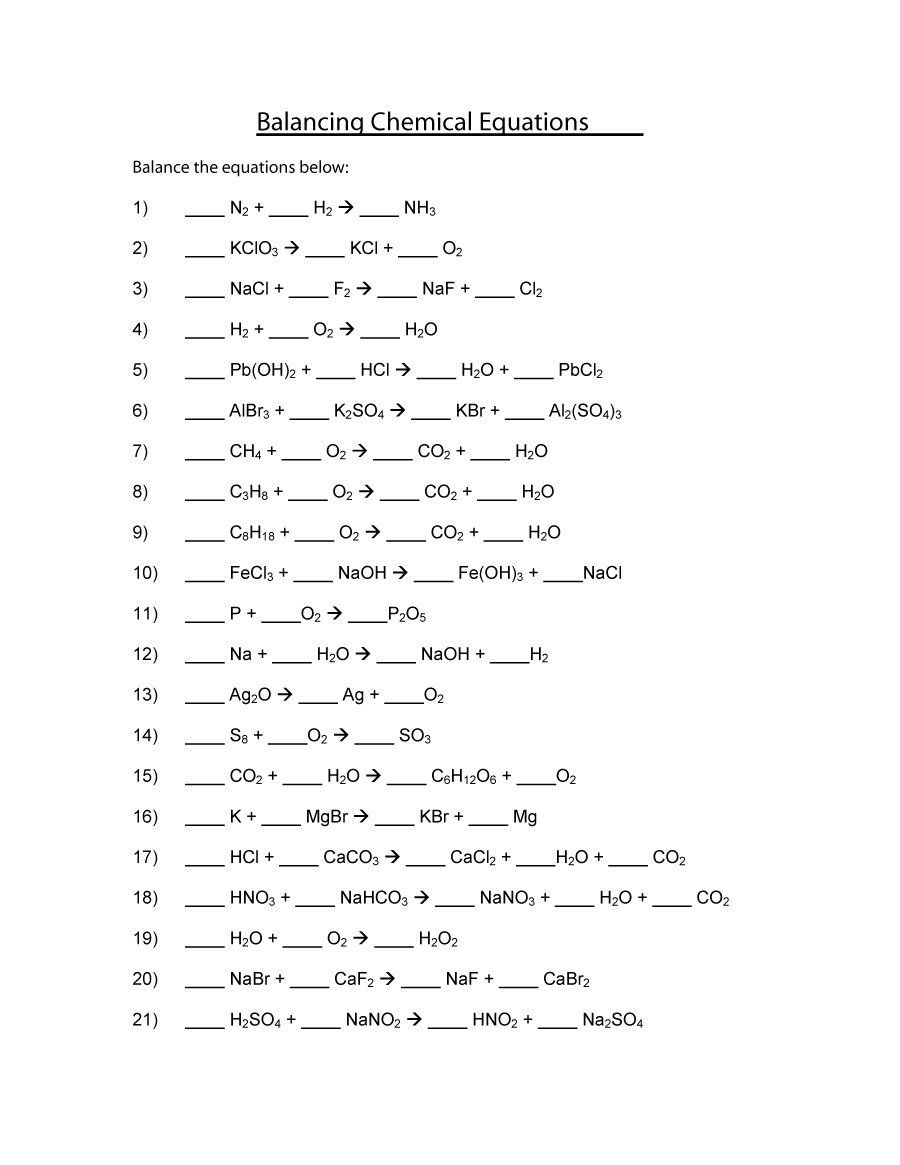
4. Balance Equations Step by Step

Balancing chemical equations is key to ensuring the law of conservation of mass is upheld. Here's a systematic approach:
- Count the atoms of each element in the reactants and products.
- Start with the most complex molecule and balance it first.
- Use coefficients (numbers placed in front of the formula) to balance.
- Recheck each element to ensure all are balanced.
- If the equation is still not balanced, revisit steps 2-4 or use a balancing strategy like the half-reaction method.
For example, to balance the equation for the reaction between hydrogen and oxygen:
H2 + O2 → H2O
First, count atoms:
- H: 2 on the left, 2 on the right
- O: 2 on the left, 1 on the right
Since we have an odd number of O on the right, start balancing by placing a 2 in front of H2O:
H2 + O2 → 2H2O
Now, balance H:
2H2 + O2 → 2H2O
🧮 Note: Balance equations in the simplest whole-number form possible, and always ensure that both sides have the same number of atoms for each element.
5. Practice with Real-world Examples

Apply your knowledge to real-world scenarios. Here are a few practice questions:
- When iron rusts, it reacts with oxygen in the air to form iron(III) oxide. Write the balanced equation for this rusting process.
- Ammonia can be synthesized by reacting nitrogen with hydrogen. Write the balanced chemical equation for this synthesis.
- Magnesium reacts with hydrochloric acid to form magnesium chloride and hydrogen gas. Write and balance this equation.
By working through these examples, you'll reinforce your understanding of word equations and improve your proficiency.
Wrapping Up

Concluding our exploration of tips for mastering chemistry word equations, we've covered key strategies such as understanding basic chemical reactions, familiarizing with chemical symbols, identifying keywords, step-by-step balancing, and real-world practice. This holistic approach not only enhances your problem-solving skills but also deepens your understanding of chemical reactions at a fundamental level. Remember, consistent practice is the key to proficiency in any field, especially chemistry.
Why is it important to balance chemical equations?

+
Balancing chemical equations is essential to ensure that the law of conservation of mass is upheld, meaning the number of atoms of each element must be the same on both the reactant and product sides.
How do keywords help in writing chemical equations?

+
Keywords like “burns,” “reacts,” “decomposes,” or “replaces” indicate the type of reaction, which can guide you in predicting the products and reactants involved.
Can I use fractions or decimals to balance chemical equations?

+
Generally, chemical equations are balanced using whole numbers to represent the smallest ratio of reactants and products, though fractions can be used temporarily for intermediates during balancing.
What’s the role of real-world examples in learning chemical equations?
+
Real-world examples bridge theoretical knowledge with practical applications, making abstract concepts more tangible and relatable, which in turn enhances learning and retention.
Are there any common mistakes students make when balancing equations?

+
Common mistakes include forgetting to count all atoms of an element, balancing only one side of the equation, or not using the smallest possible whole-number coefficients. Also, sometimes students overlook the conservation of mass.



