Solubility Graphs Worksheet: Your Answer Key Awaits

In the world of chemistry, understanding solubility is akin to unlocking a treasure trove of molecular interactions. Solubility graphs are powerful tools that chemists use to predict, analyze, and visualize how substances dissolve in solvents under various conditions. This comprehensive guide will delve into the intricacies of solubility graphs, providing an answer key for a solubility worksheet, and ensuring that both students and professionals can better grasp this fundamental concept.
Understanding Solubility Graphs
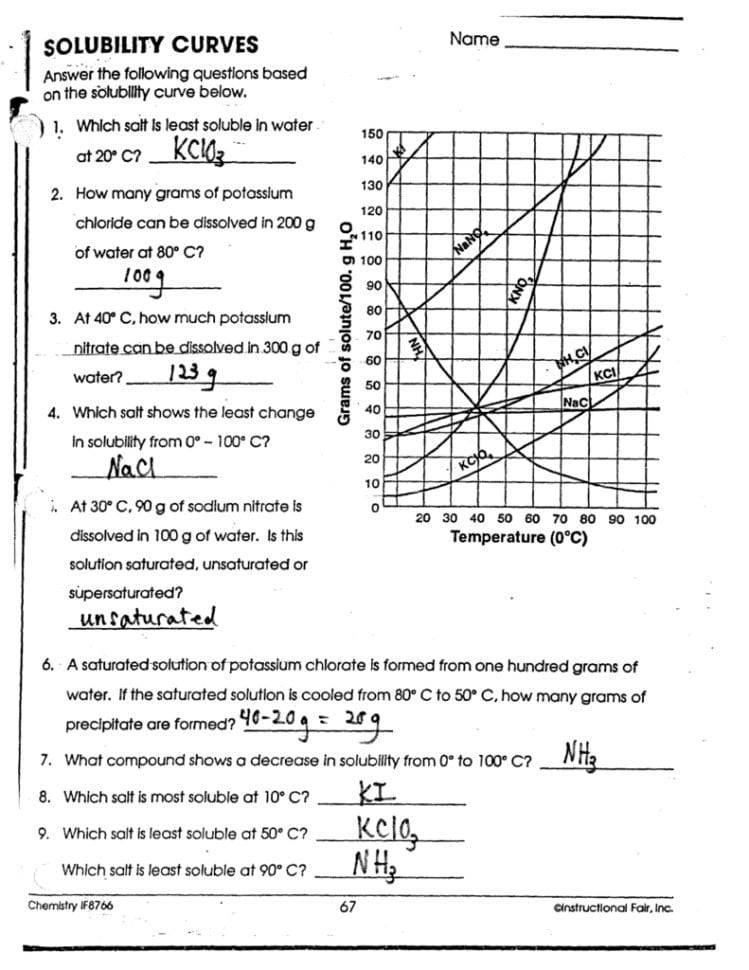
Solubility graphs, also known as solubility curves, are graphical representations of how the solubility of a substance changes with temperature. These graphs are critical because:
- They illustrate the relationship between solubility and temperature.
- They allow for the prediction of whether a solution is saturated, unsaturated, or supersaturated at given conditions.
- They help in determining the solubility product constant (Ksp) for sparingly soluble salts.

How to Read a Solubility Graph

A typical solubility graph will have temperature on the x-axis and solubility on the y-axis. Here’s how to interpret the information:
- Y-Intercept: This represents the solubility at 0°C.
- Slope of the Line: Indicates how the solubility changes with temperature. An upward slope means solubility increases with temperature, while a downward slope indicates the opposite.
- Saturation Points: At any point on the line, the solution is saturated. Below the line, it’s unsaturated, and above it, supersaturated.
Components of Solubility Curves
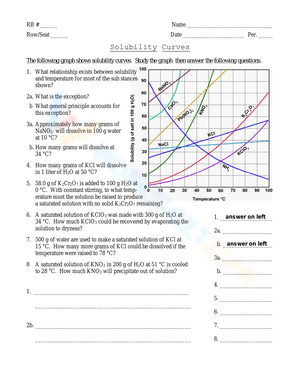
When dealing with solubility graphs, several key components need consideration:
| Component | Description |
|---|---|
| Curve | The actual line on the graph showing solubility vs. temperature. |
| Intersection Points | Where the solubility curve meets lines indicating different temperature points. |
| Saturation | The state of the solution at various points on or off the curve. |
| Temperature Change | How solubility changes with a change in temperature. |

Practical Applications
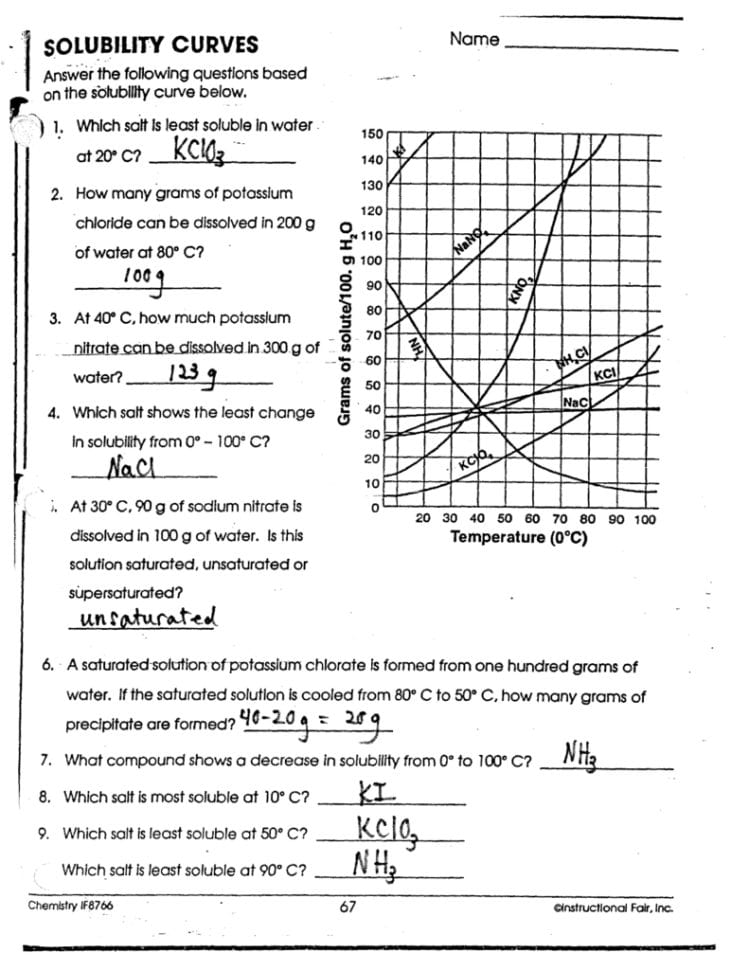
Solubility graphs are not just theoretical constructs; they have practical applications:
- Chemical Analysis: To determine if a solution can precipitate out of a given compound at specific conditions.
- Industrial Processes: For designing cooling or heating systems in chemical manufacturing.
- Environmental Science: To predict pollutant behavior in natural water systems.
Your Solubility Graph Worksheet: Answer Key
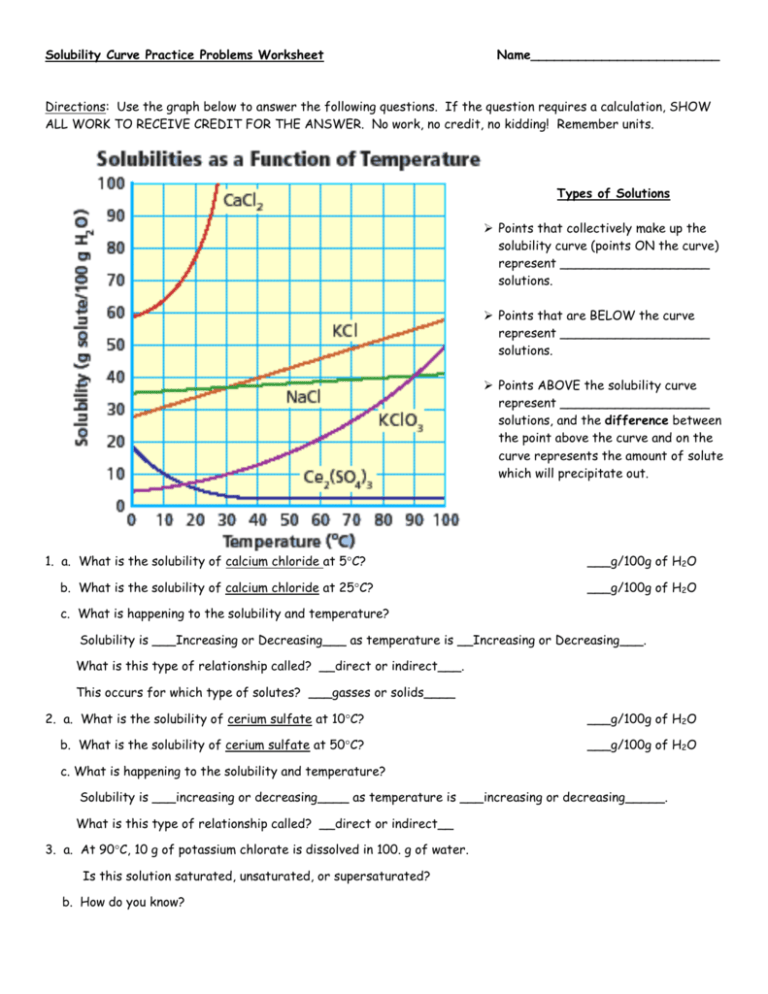
Here are the answers to common questions from a typical solubility graph worksheet:
- Q1. At what temperature does the solubility of potassium nitrate (KNO3) equal 50g per 100g of water?
- Q2. What is the solubility of sodium chloride (NaCl) at 50°C?
- Q3. Which compound shows the greatest increase in solubility with temperature?
- Q4. If you add 40g of sodium nitrate (NaNO3) to 100g of water at 30°C, is the solution saturated, unsaturated, or supersaturated?
- Q5. Estimate the solubility of potassium chlorate (KClO3) at 70°C.
From the graph, the solubility of KNO3 reaches 50g/100g H2O at approximately 50°C.
At 50°C, NaCl has a solubility of around 35g/100g of water.
Based on the graph, potassium nitrate (KNO3) exhibits the steepest slope, indicating the greatest change in solubility with temperature.
The graph indicates that NaNO3 has a solubility of about 45g/100g at 30°C. Adding 40g would result in an unsaturated solution.
📌 Note: Remember, supersaturated solutions are unstable and can precipitate out the excess solute with a minor disturbance.
The solubility of KClO3 at 70°C, according to the graph, is approximately 160g/100g of water.
In summary, solubility graphs are essential tools in chemistry for predicting and understanding the behavior of substances in solution. By reading these graphs correctly, one can determine the solubility at different temperatures, predict precipitation conditions, and manage industrial processes effectively. This knowledge not only equips chemists with practical skills but also enriches our understanding of molecular interactions at a fundamental level.
Why are solubility graphs important?

+
Solubility graphs are crucial for predicting how much of a substance will dissolve under different temperature conditions, which is vital for chemical analysis, industrial processes, and environmental monitoring.
Can solubility decrease with temperature?
+
Yes, for some substances, solubility decreases with temperature, such as with certain gases or compounds like cerium sulfate (Ce(SO4)2).
What does a flat solubility curve indicate?

+
A flat solubility curve indicates that the solubility of the substance is relatively constant with changes in temperature, suggesting that other factors like pressure or solute concentration might influence solubility more.