5 Ways to Separate Mixtures for Students
One of the fundamental principles in chemistry and physics is the concept of separation. Separating mixtures is an essential skill for students to understand the composition of substances, enabling further study and analysis. Here, we delve into five common methods used to separate mixtures, each catering to different physical and chemical properties of the components involved.
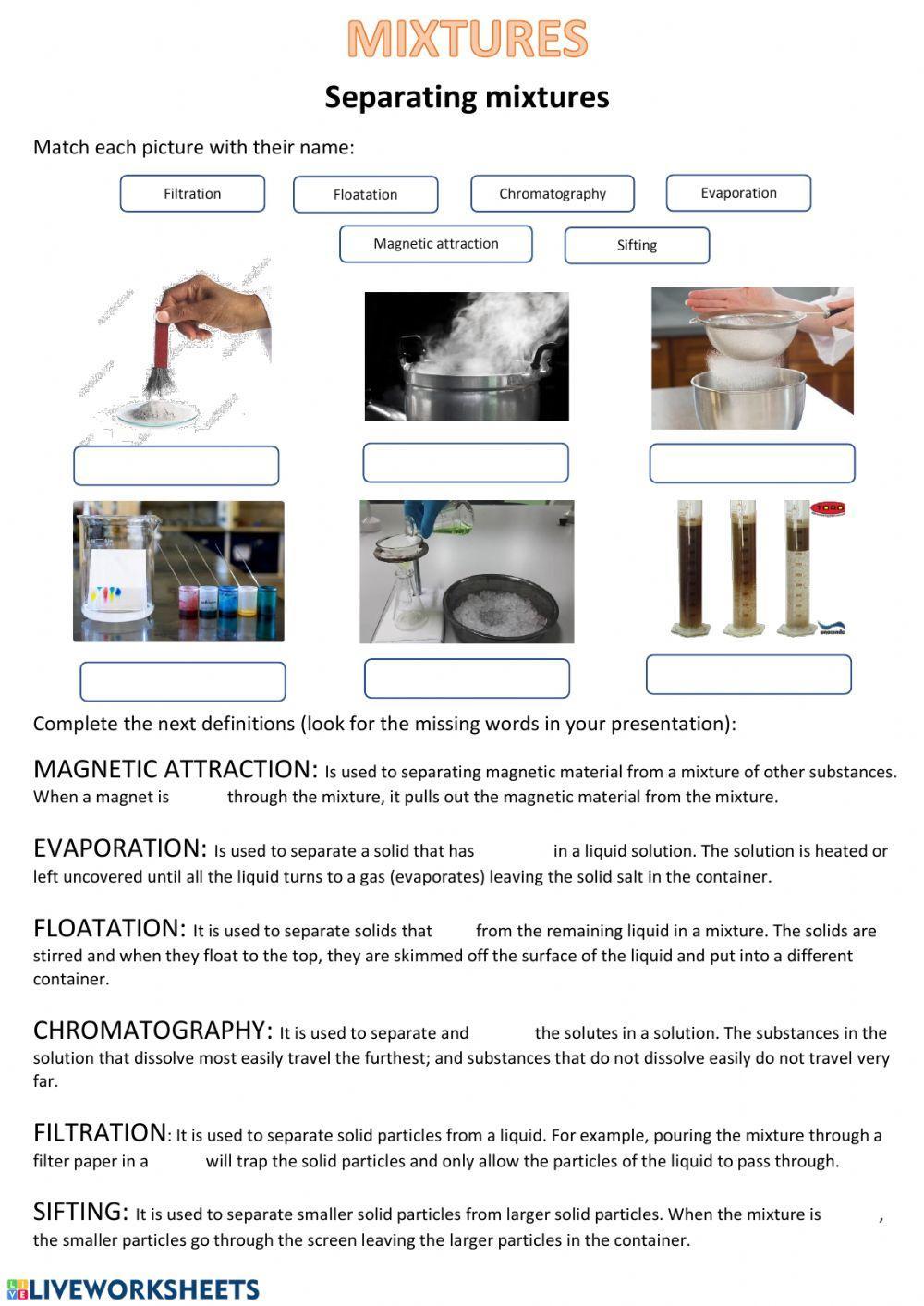
Filtration


Filtration is often one of the first separation techniques students encounter. This method exploits the difference in the sizes of particles within a mixture. Here’s how you can apply filtration:
- Choose the right filter: Select a filter paper or medium with pore sizes that allow the liquid or gas to pass through while retaining the solid particles.
- Set up your apparatus: Typically, this involves placing a funnel with filter paper over a container where the filtrate (clear liquid) will collect.
- Pour the mixture: Slowly pour the mixture onto the filter paper. The larger particles are trapped on the surface while the smaller ones or dissolved substances pass through.
Filtration is ideal for:
- Separating solids from liquids in a suspension.
- Removing impurities from a liquid.
- Extracting substances from biological samples.
💡 Note: For mixtures where the solid particles are very fine, you might need to use vacuum filtration or centrifugation to achieve better separation results.
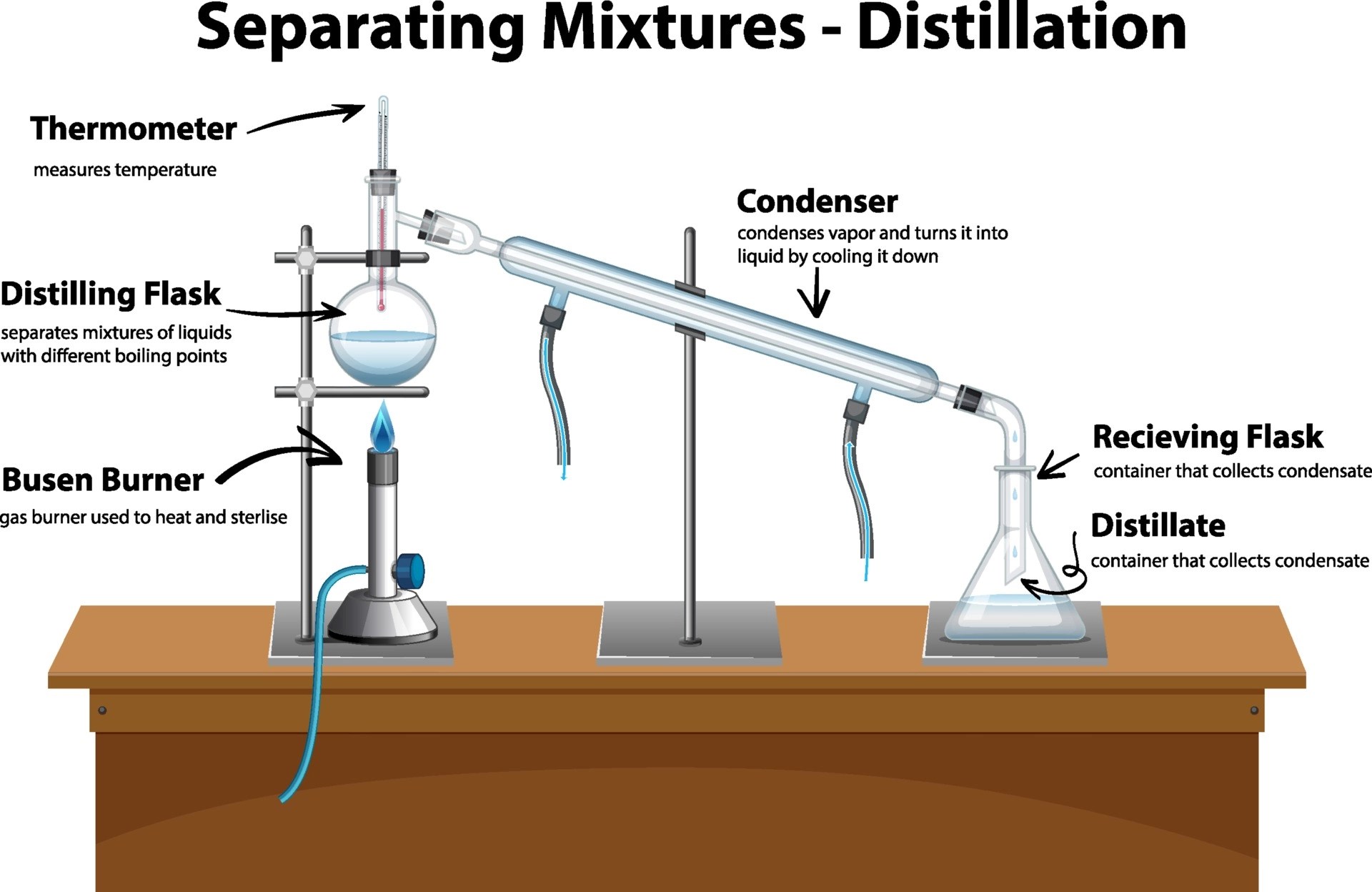
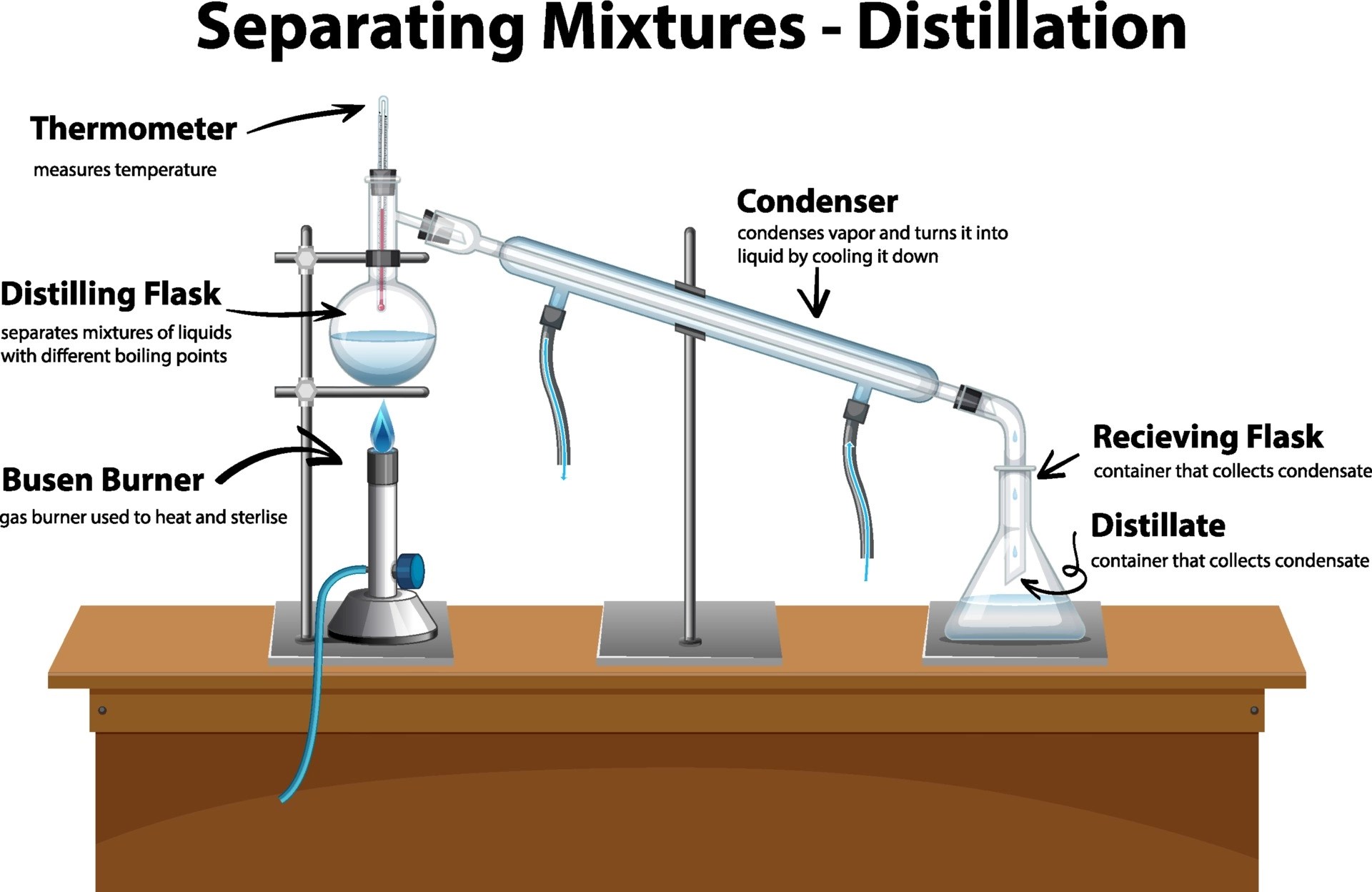
Distillation


When dealing with liquids that have different boiling points, distillation proves to be an effective method:
- Heat the mixture: The mixture is heated in a distillation flask until it reaches the boiling point of the most volatile component.
- Collect the distillate: Vapors rise into the condenser where they are cooled and then collected.
- Repeat if necessary: If there are multiple liquids with similar boiling points, fractional distillation can be used for finer separation.
Distillation is useful for:
- Refining crude oil into various fractions like petrol, kerosene, etc.
- Separating alcohol from water or other substances in the brewing industry.
- Purifying organic compounds for laboratory analysis.
🧪 Note: Ensure proper disposal of any distilled substances as they might be hazardous.
Centrifugation


Centrifugation uses centrifugal force to separate substances, typically when gravity alone isn’t sufficient:
- Select the appropriate speed and duration: These parameters depend on the mixture’s properties, specifically the density and size of particles.
- Prepare your samples: Place the mixture in test tubes or centrifuge bottles evenly balanced across the rotor.
- Run the centrifuge: Once the machine is running, the heavier particles move towards the bottom due to the centrifugal force, separating them from lighter substances.
Applications include:
- Separating blood components for medical diagnostics.
- Clarification of liquids in industries like pharmaceuticals.
- Isolating DNA or proteins from cell suspensions in molecular biology.
⚙️ Note: Balancing the centrifuge is crucial to prevent machine damage and ensure sample integrity.
Chromatography

Chromatography separates mixtures based on different interaction rates with a stationary and a mobile phase:
- Choose the type: There are various types including paper, thin-layer, column, gas, and high-performance liquid chromatography (HPLC).
- Prepare the sample: The mixture to be separated is applied onto the stationary phase.
- Introduce the mobile phase: As this phase moves, components of the mixture move at different speeds, separating them based on their interaction with the stationary phase.
Chromatography excels in:
- Identifying and analyzing chemical mixtures.
- Studying pigments in plants.
- Forensic analysis for separating ink in written documents.
🔬 Note: Ensure the stationary phase is compatible with the solvent used to prevent degradation or contamination.
Magnetic Separation


This method utilizes the magnetic properties of materials to segregate them from a mixture:
- Apply a magnetic field: A magnet is moved over the mixture or the mixture is passed over a magnetized surface.
- Separate the magnetized material: If the materials to be separated have differing magnetic susceptibilities, one will be attracted to the magnet.
Useful applications include:
- Extracting iron filings from other non-magnetic powders.
- Cleaning up oil spills with magnetic absorbents.
- Purifying water by removing magnetic contaminants.
🔍 Note: Magnetic separation can be enhanced with techniques like high-intensity magnetic separation for finer or weakly magnetic particles.
To conclude, understanding how to separate mixtures is not only crucial for laboratory experiments but also has practical applications in industries ranging from pharmaceuticals to mining. Each method provides unique advantages tailored to specific properties of substances. Students can explore these techniques to gain insights into the behavior of matter and its interaction with different forces, paving the way for advancements in science and technology.
Can any mixture be separated by all these methods?

+
Not all mixtures can be separated using every method. The choice of method depends on the physical properties of the components in the mixture, such as boiling point, solubility, magnetic properties, or particle size.
Why is it important to learn how to separate mixtures?
+
Separating mixtures is essential in understanding the composition of materials, which is crucial for various scientific disciplines and industrial applications. It helps in purifying substances, analyzing components, and even in environmental management like pollution control.
What is the most common separation technique?

+
Filtration is perhaps the most frequently used due to its simplicity, cost-effectiveness, and wide applicability, especially in the initial stage of separating solids from liquids or gases.