Osmosis and Tonicity Worksheet: Boost Your Biology Skills

Osmosis and tonicity are fundamental concepts in biology, playing crucial roles in how cells interact with their environments. Understanding these processes can significantly enhance one's grasp of biological systems, from the microscale of cellular interactions to the macroscale of physiological responses in living organisms. This comprehensive guide delves into osmosis, tonicity, and how they apply to cellular biology, offering insights and practical knowledge to enhance your biology skills.
The Basics of Osmosis

Osmosis refers to the movement of water molecules across a semipermeable membrane from an area of lower solute concentration to an area of higher solute concentration. Here’s how you can remember this:
- The movement is from a hypotonic (low solute concentration) environment to a hypertonic (high solute concentration) environment.
- The goal of osmosis is to achieve equilibrium of water concentration on both sides of the membrane.
🌟 Note: Osmosis does not require energy from the cell; it’s a passive process.
Understanding Tonicity

Tonicity describes the concentration of solutes in solutions and how they influence the net movement of water across a membrane. Here are the key types of tonicity:
| Type of Solution | Characteristics |
|---|---|
| Isotonic | Equal solute concentration inside and outside the cell; no net water movement. |
| Hypotonic | Lower solute concentration outside than inside the cell; water enters the cell, causing it to swell or burst. |
| Hypertonic | Higher solute concentration outside than inside the cell; water leaves the cell, causing it to shrivel. |

Applying Osmosis and Tonicity in Cellular Biology

The principles of osmosis and tonicity are not just academic; they have practical implications in:
- Medical Treatments: Understanding tonicity is crucial in the formulation of IV fluids.
- Agricultural Practices: Proper hydration of plant cells to maintain turgor pressure.
- Biological Research: Experimental design in cell biology often relies on these principles.
Here are some key applications:
- Red Blood Cells: Exposure to a hypertonic solution causes cells to shrivel (crenate), while hypotonic solutions make them swell and potentially burst (hemolyze).
- Plant Cells: The presence of a cell wall allows plant cells to withstand hypotonic environments by maintaining a balance against turgor pressure.
🌱 Note: Plant cells can regulate their internal tonicity through mechanisms like stomatal closure to prevent excessive water loss or uptake.
Worksheet on Osmosis and Tonicity
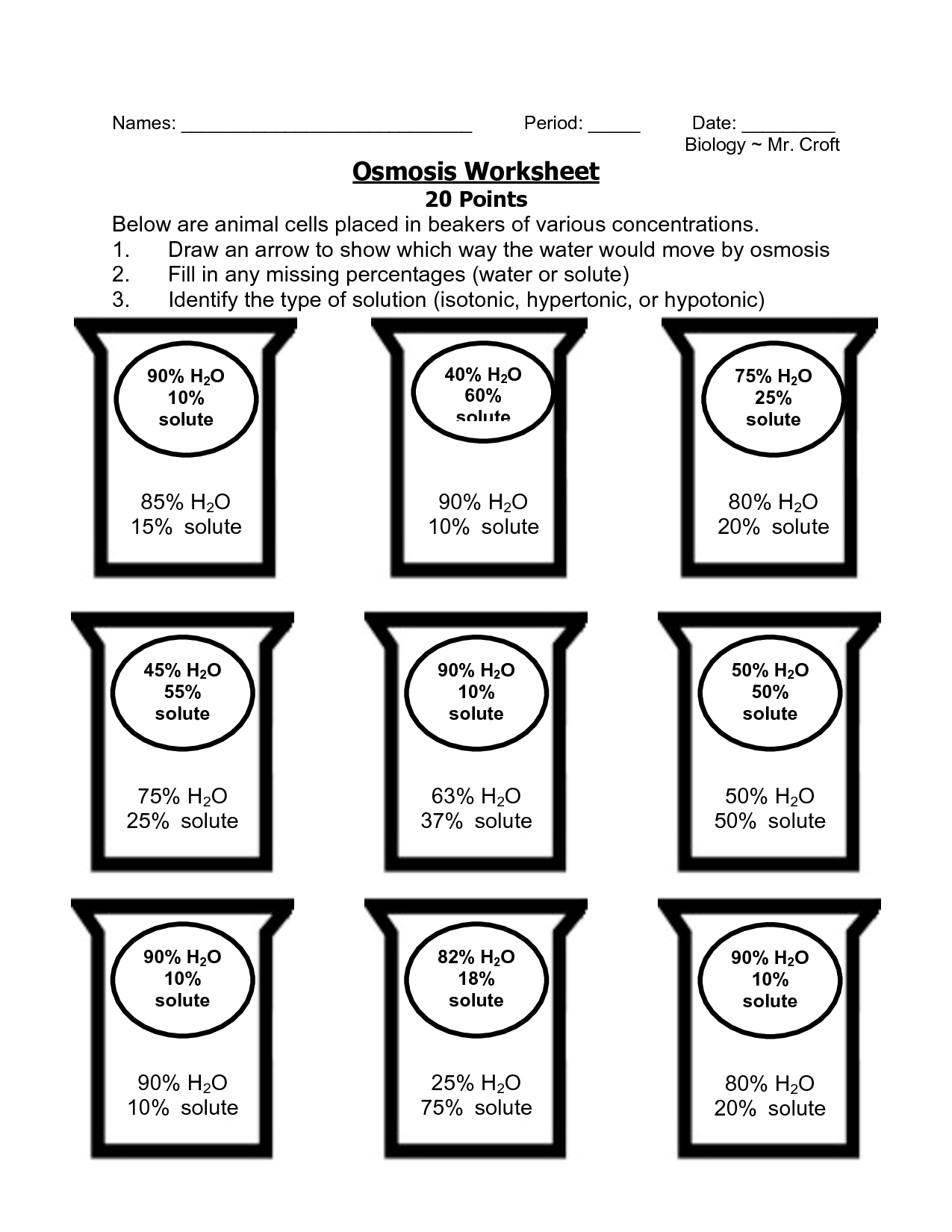
To help solidify your understanding, here’s a worksheet designed to test and expand your knowledge on these topics:
- Label the following conditions for cells:
- Cell in freshwater (hypotonic, hypertonic, isotonic)
- Cell in seawater (hypotonic, hypertonic, isotonic)
- Explain why a patient with low blood pressure might be administered a hypertonic solution.
- Describe how a plant cell manages water uptake in various environments:
- When the soil is well-hydrated
- During a drought
- Draw and label the effects of osmosis on animal and plant cells in different solutions.
🧪 Note: Practice makes perfect, so use simulations or labs to visually understand osmosis and tonicity.
This exploration of osmosis and tonicity not only aids in mastering these biological processes but also opens up a window into understanding how life functions at its core. These principles are fundamental to how cells maintain homeostasis, how organisms adapt to their environment, and how medicine can address cellular issues. By grasping these concepts, you'll be better prepared for complex biological scenarios and excel in your biology studies.
What is the difference between osmosis and diffusion?

+
Osmosis is the movement of water across a selectively permeable membrane, whereas diffusion is the movement of any substance from an area of high concentration to an area of low concentration, until equilibrium is reached.
Can osmosis occur without a semipermeable membrane?

+
No, osmosis specifically refers to the movement of water across a semipermeable membrane. Without this barrier, the term ‘diffusion’ would be more appropriate for the general movement of water molecules.
How does tonicity affect plant cells differently than animal cells?

+
Plant cells have a cell wall that provides structural support. In hypotonic environments, they can swell without bursting due to the rigid cell wall, a condition known as turgor pressure. Animal cells, lacking this support, can burst when placed in hypotonic solutions.