Ionic Bonding Worksheet: Master Chemistry with Ease
Understanding ionic bonding is crucial for students diving into the vast world of chemistry. It's like unlocking a door to understanding how substances are built at their most fundamental level. In this blog post, we'll guide you through creating an Ionic Bonding Worksheet that will help you or your students master this essential concept with ease. Whether you're a teacher looking to supplement your curriculum or a student aiming to deepen your grasp of chemistry, this post has something for you.
The Basics of Ionic Bonding
Before we delve into crafting a worksheet, let's briefly recap what ionic bonding is:
- Definition: Ionic bonding is the complete transfer of valence electrons between atoms, resulting in the formation of ions and the creation of ionic compounds.
- Key Points:
- Typically involves metals bonding with non-metals.
- The metal atoms lose electrons to become positively charged cations.
- Non-metals gain these electrons to become negatively charged anions.
- The resulting electrostatic attraction holds the ions together in a crystalline structure.
🔍 Note: Ionic bonds are not as strong in the liquid phase or in solution, as ions are no longer fixed in their lattice positions.
Creating an Ionic Bonding Worksheet

Worksheet Components

When designing your worksheet, consider incorporating the following elements:
1. Theoretical Questions
These questions help students understand the concept:
- What is the difference between an ionic and covalent bond?
- Why do metals and non-metals form ionic bonds?
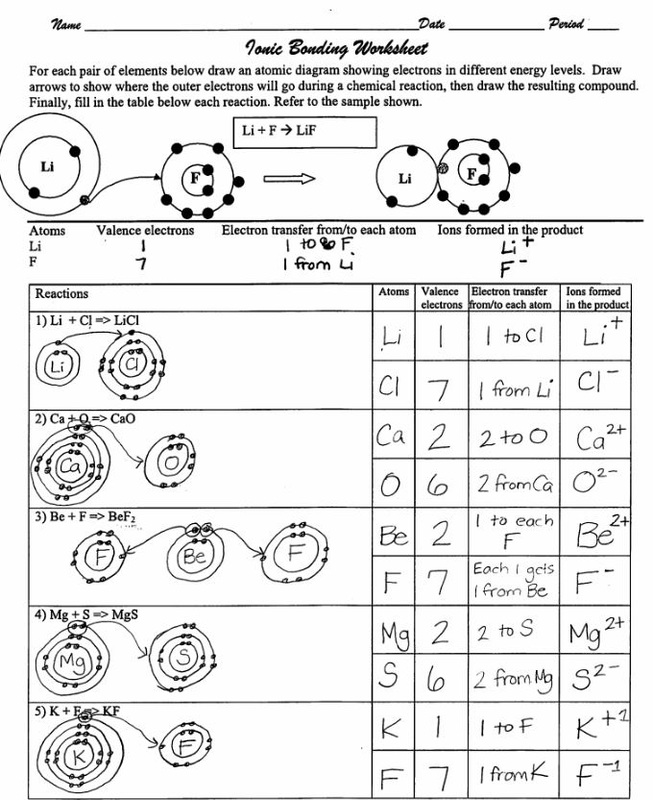
2. Electron Configuration
Including sections that prompt students to draw electron configurations for elements involved in ionic bonding:
- Draw the electron configuration for Sodium and Chlorine atoms.
- Show the transfer of electrons to form Sodium Chloride (NaCl).
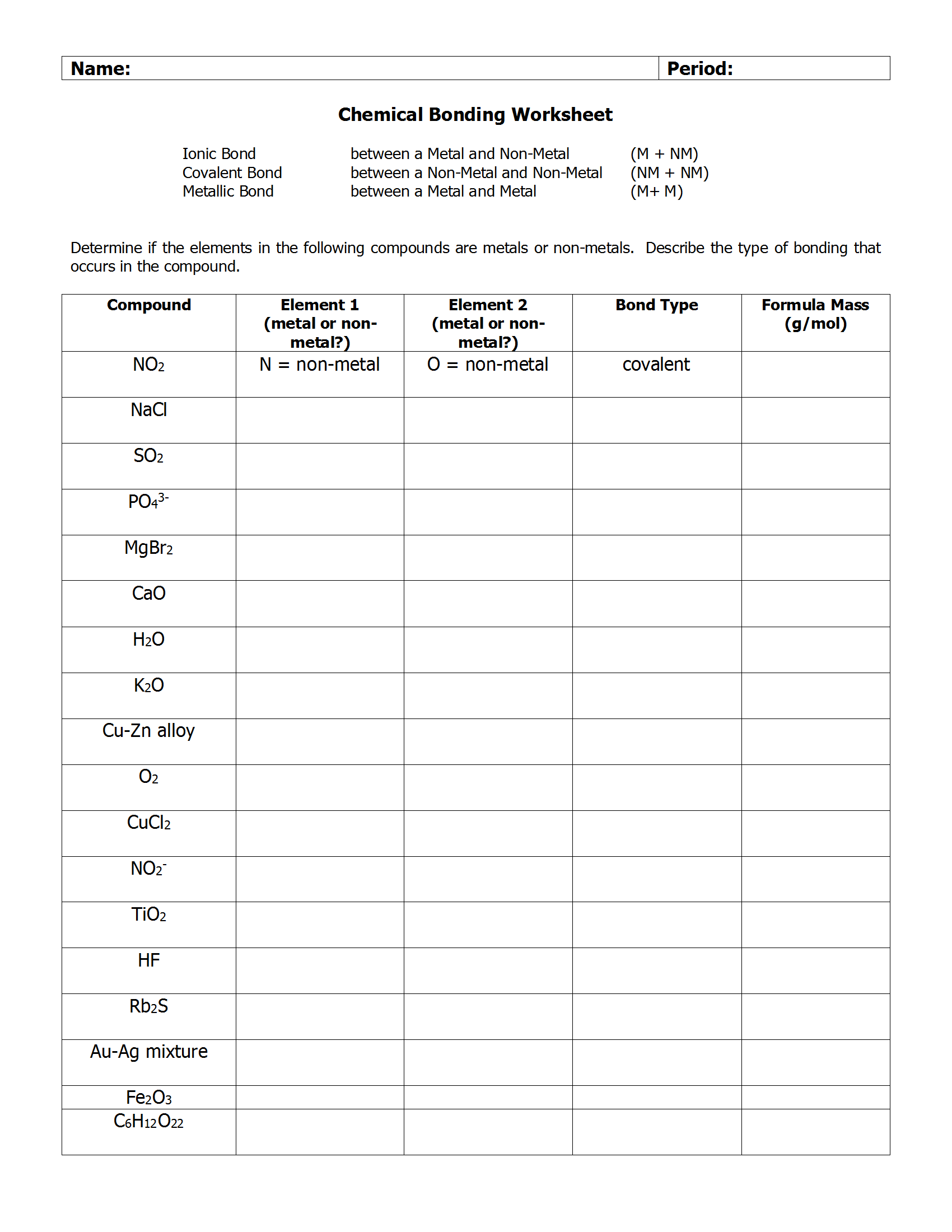
3. Formation of Ionic Compounds
Have exercises where students must combine different ions to form compounds:
| Element | Charge | Compound Formed |
|---|---|---|
| Calcium | Ca2+ | Calcium Oxide |
| Oxygen | O2- |

🧐 Note: Ensure the worksheet progresses from simple compounds like NaCl to more complex ones like BaSO4 or Fe2O3.
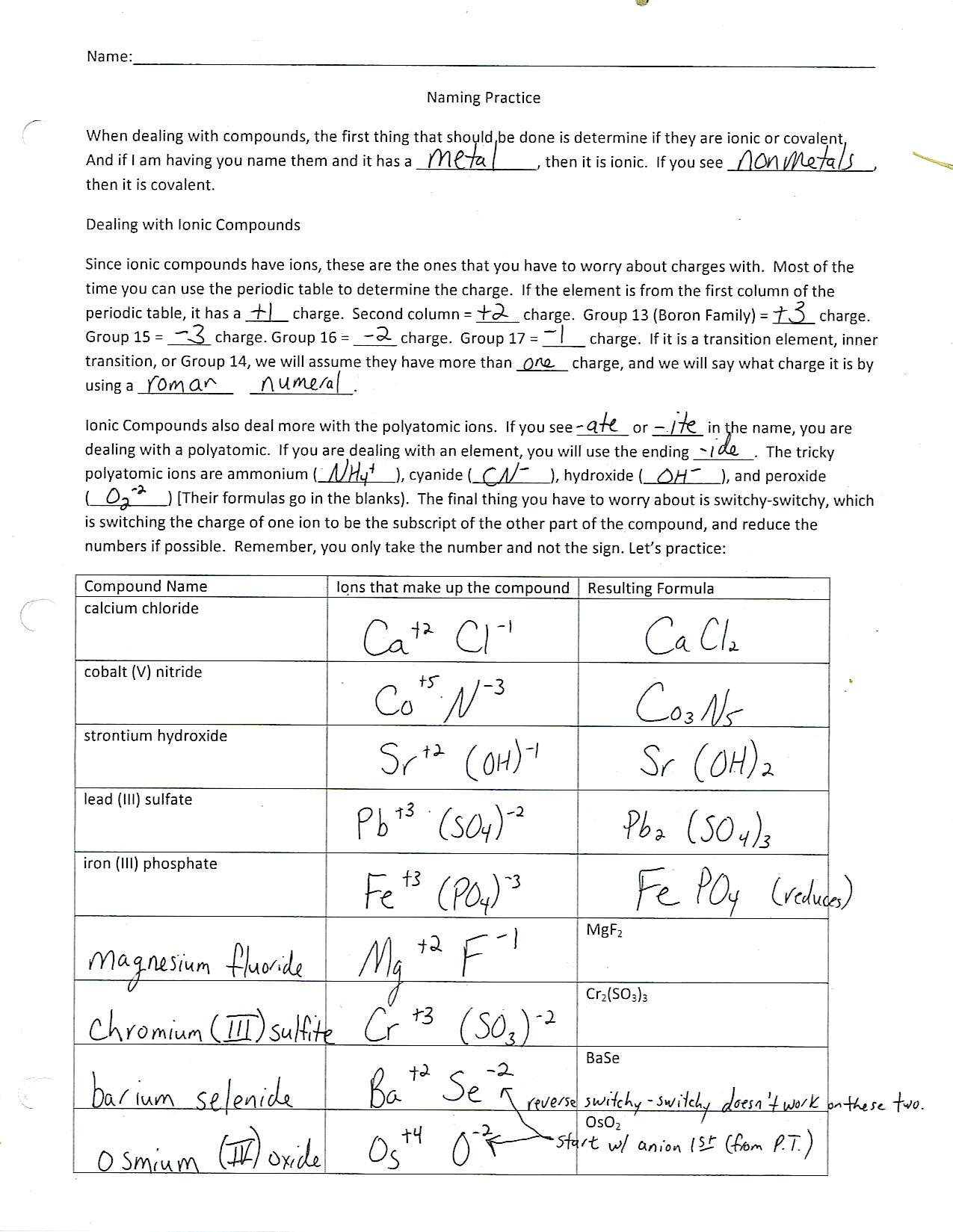
4. Naming Ionic Compounds
Include exercises where students practice naming ionic compounds based on their constituent elements:
- What is the name of K2SO4?
- Write the formula for Magnesium Nitrate.
5. Properties of Ionic Compounds
Explore the physical and chemical properties of ionic compounds:
- Why are ionic compounds generally brittle?
- Explain why ionic compounds conduct electricity when dissolved in water.
How to Structure Your Worksheet

Here is a step-by-step guide to creating a structured ionic bonding worksheet:
- Introduction: Start with an overview or introduction to ionic bonding.
- Conceptual Questions: Place theoretical questions early to set the stage for understanding.
- Electron Configurations: Follow with activities on electron configurations to solidify the concept of electron transfer.
- Ionic Compound Formation: Students should then practice creating compounds.
- Naming: Introduce the nomenclature section where students apply their understanding of ionic bonding to name compounds.
- Properties: Conclude with questions about the properties of ionic compounds to connect theory with practical chemistry.
- Summary or Review: End with a summary or review section to reinforce key points.
⚠️ Note: Provide clear instructions for each section to help guide student's thought processes.
Conclusion
As we’ve seen, an Ionic Bonding Worksheet can significantly aid in grasping the complexities of chemical bonds. By systematically approaching the topic from electron configurations to naming compounds and understanding their properties, students gain a comprehensive understanding of ionic bonding. Such a worksheet not only fosters problem-solving skills but also helps in visualizing the atomic interactions that govern our chemical world. Remember, the key to mastering chemistry is practice, so encourage students to work through this worksheet, and they’ll find themselves on solid ground when it comes to ionic bonding.
Why are ionic bonds strong in solids but weak in solution?

+
Ionic bonds are strong in solid compounds because the ions are held in a lattice structure by strong electrostatic forces. When dissolved in a solvent or melted, these forces are weakened as the ions can move apart from each other, leading to weaker bonds in solution.
How do you determine the charge of an ion?

+
The charge of an ion is determined by how many electrons an atom has to gain or lose to achieve a stable electron configuration, typically an octet (8 electrons in the outer shell). For example, Sodium (Na) loses one electron to become Na+, while Chlorine (Cl) gains one to become Cl-.
Can ionic bonds form between two non-metals?

+
Typically, ionic bonds form between a metal and a non-metal due to the significant difference in electronegativity. While it’s uncommon, some compounds like ammonium nitrate (NH4NO3) can form through ionic bonding between two polyatomic ions, where one acts as a pseudo-metal due to its positive charge.