5 Key Milestones in Atomic Theory Development

The journey into the intricate world of atomic theory is as fascinating as it is fundamental, with its understanding reshaping the fields of physics, chemistry, and material science. Let's delve into five pivotal milestones that have sculpted our comprehension of the atom.
Democritus and the Concept of Atomos

More than two millennia ago, a profound philosophical thought experiment conducted by the Greek philosopher Democritus laid the foundational stone of atomic theory. He proposed the idea that if you keep cutting a material, you would eventually reach a point where it could no longer be divided. These indivisible units, he termed, were "atomos" or atoms. Although lacking empirical evidence, Democritus’s vision suggested that matter is not infinitely divisible, a concept that would persist through centuries.
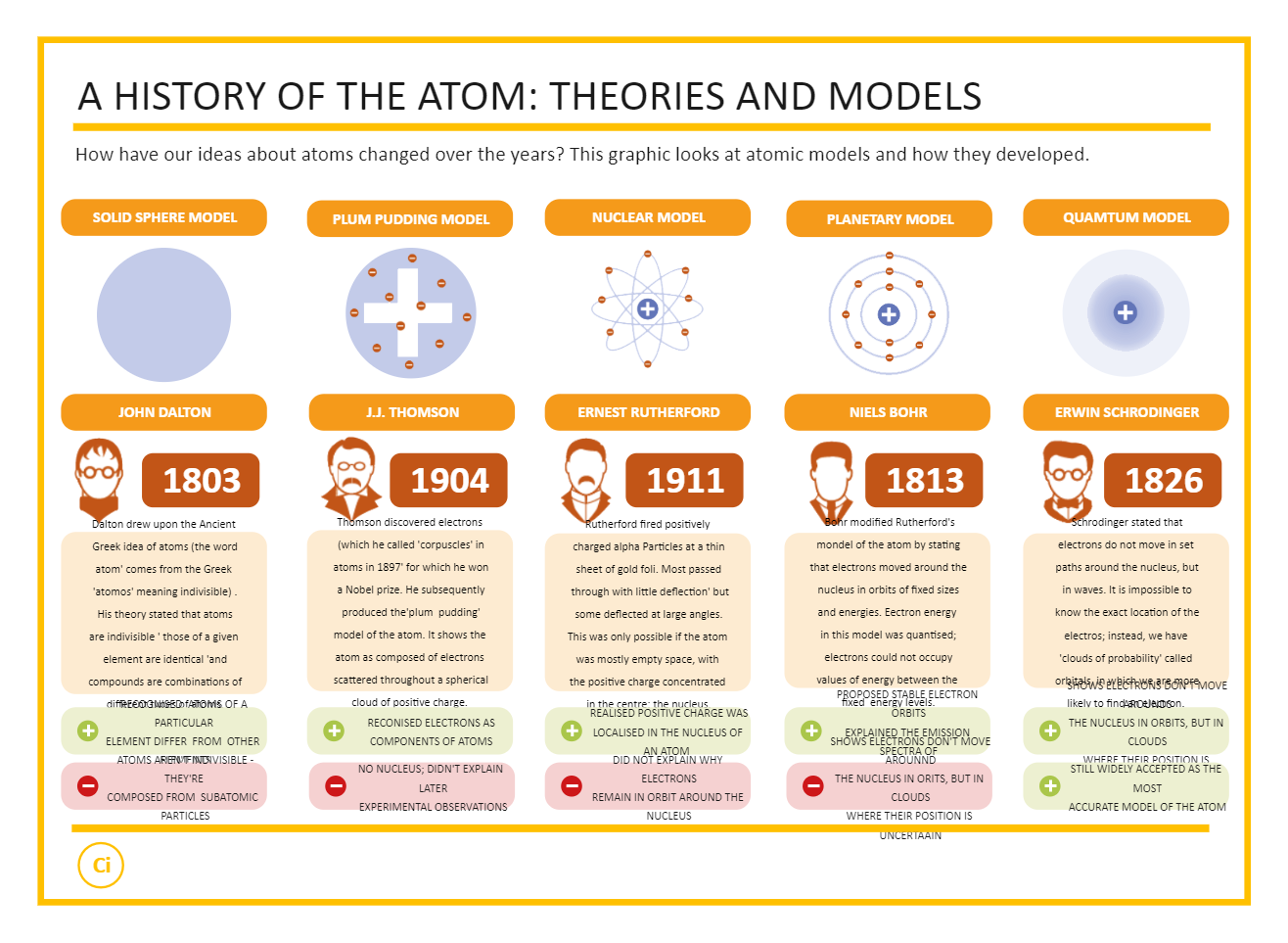
John Dalton's Modern Atomic Theory

It wasn’t until the early 19th century, during the age of Enlightenment, that atomic theory made a leap from philosophy to science with John Dalton’s Modern Atomic Theory in 1808. Dalton:
- Hypothesized that elements are composed of minute, indivisible particles known as atoms.
- Posited that all atoms of an element are identical in mass and properties, but different from atoms of other elements.
- Explained that chemical reactions involve rearrangements of these atoms.
- Introduced the law of multiple proportions, which later became crucial in the understanding of molecular stoichiometry.
His work not only explained experimental data but also led to the idea of relative atomic masses, crucial for the progress of chemistry.
J.J. Thomson and the Discovery of the Electron

In 1897, J.J. Thomson ushered in the next phase of atomic theory by discovering the electron, a constituent particle of the atom. His experiments with cathode rays paved the way for:
- Proving that atoms were not indivisible, as Dalton had theorized.
- Formulating the Plum Pudding Model, envisioning atoms as positively charged spheres with negatively charged electrons embedded within.
Ernest Rutherford's Nuclear Model

The atomic model took another leap forward with Ernest Rutherford in 1911. His gold foil experiment revealed:
- Most of the atom’s mass and all of its positive charge reside in a tiny, dense central core, which he called the nucleus.
- Electrons orbit around this nucleus, much like planets orbiting the sun.
- The atom is largely composed of empty space, challenging the previous understanding of atomic structure.
Niels Bohr's Quantum Model

Building upon Rutherford's model, Niels Bohr proposed in 1913 a theory where:
- Electrons move in fixed orbits or energy levels.
- Energy is emitted or absorbed when electrons jump between these levels.
- He introduced the concept of quantized energy, aligning atomic structure with quantum mechanics.
His model brought a new depth to our understanding, explaining atomic spectra, chemical bonding, and stability in atoms, earning him the Nobel Prize in Physics in 1922.
The development of atomic theory has revolutionized our understanding of the material world, from providing explanations for phenomena in chemistry to uncovering the fundamental nature of energy and matter in physics. Here are some noteworthy points:
⚛️ Note: The journey of atomic theory is not a linear progression but rather a dialectic evolution where each new theory builds upon or challenges its predecessors.
🧪 Note: The transition from Dalton's billiard ball model to Bohr's quantum model was essential in the shift from classical to modern physics.
🔬 Note: Scientific advancement in atomic theory did not occur in isolation; it was fueled by interdisciplinary collaborations and groundbreaking experiments.
This exploration underscores the dynamic and progressive nature of scientific inquiry. With each discovery, scientists peel back another layer of the mysterious veil of the atomic world, presenting us with a more intricate view of the universe's building blocks.
Why is atomic theory important?
+
Atomic theory underpins our understanding of chemical reactions, molecular bonding, and physical laws. It’s fundamental to the fields of chemistry, physics, and materials science, affecting everything from everyday technology to the core principles of how our universe functions.
How did quantum mechanics influence atomic theory?

+
Quantum mechanics introduced the concept of quantization and wave-particle duality, which provided a more accurate description of electron behavior within atoms. It resolved discrepancies in classical atomic models and gave birth to the quantum model of the atom.
Are atoms really indivisible?

+
While originally thought to be indivisible by Democritus and Dalton, modern physics has shown that atoms consist of subatomic particles like protons, neutrons, and electrons. Atoms can be split through nuclear reactions, but this process changes their elemental identity.