5 Steps to Master Bohr Models Worksheet

The journey towards understanding the intricate world of atomic structure and electron configuration is not just a chapter in your chemistry textbook but a gateway to exploring how the world at its most fundamental level works. The Bohr model, introduced by Niels Bohr in 1913, provides a simplified yet elegant way to visualize the electrons in an atom and their energy levels. Mastering the Bohr models worksheet involves more than just memorizing facts; it's about engaging with the material, understanding the underlying principles, and applying them. Here are the detailed steps to guide you through this enriching experience:
1. Understand the Basics of the Bohr Model
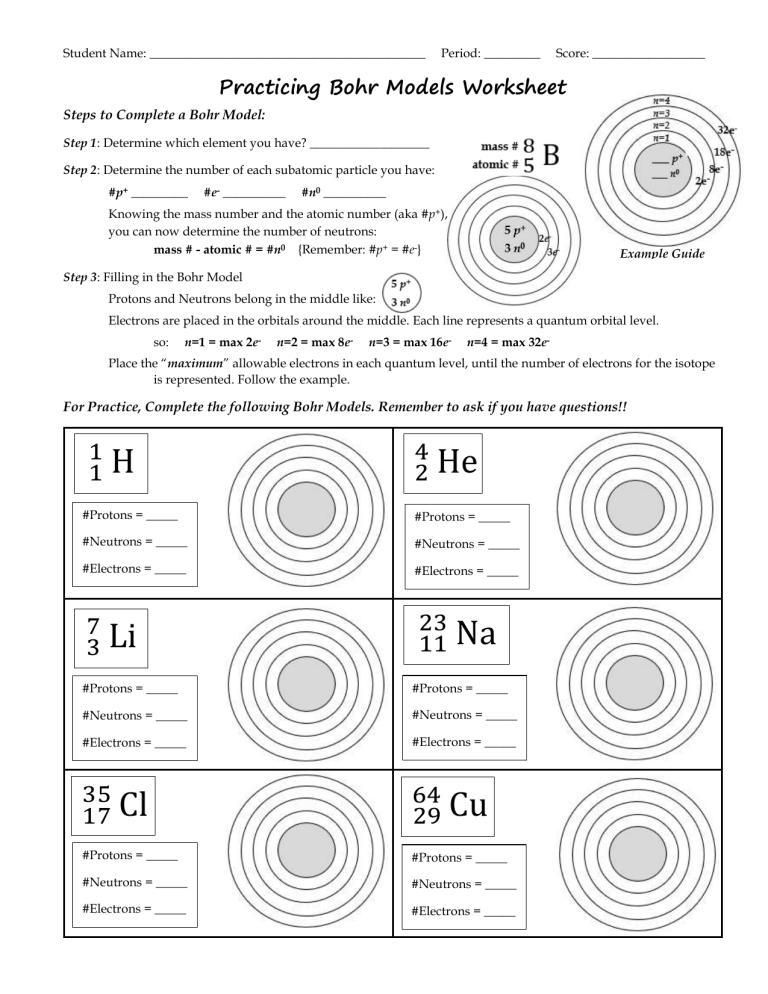
The first step to mastering the Bohr models worksheet is to solidify your understanding of what a Bohr model is and its components:
- Atom: The fundamental unit of matter, consisting of protons, neutrons, and electrons.
- Protons and Neutrons: Located in the nucleus, they define the element and its isotopes.
- Electrons: Orbiting the nucleus in what Bohr defined as ‘shells’ or ‘energy levels.’ These levels are quantized, meaning electrons can only exist in specific levels, not in-between.
- Electron Configuration: The arrangement of electrons in these shells, where each level can hold a specific number of electrons (2n², where n is the shell number).

Visualizing Electron Shells

Each shell corresponds to an energy level. Here’s how they are organized:
- 1st Shell (K shell): can hold up to 2 electrons.
- 2nd Shell (L shell): up to 8 electrons.
- 3rd Shell (M shell): up to 18 electrons.
- 4th Shell (N shell): up to 32 electrons, but for our purpose in understanding basic Bohr models, this often simplifies to only considering the first few shells.
🌟 Note: While the Bohr model is a simplification, it laid the groundwork for more complex quantum models of atomic structure.
2. Practice Identifying Elements
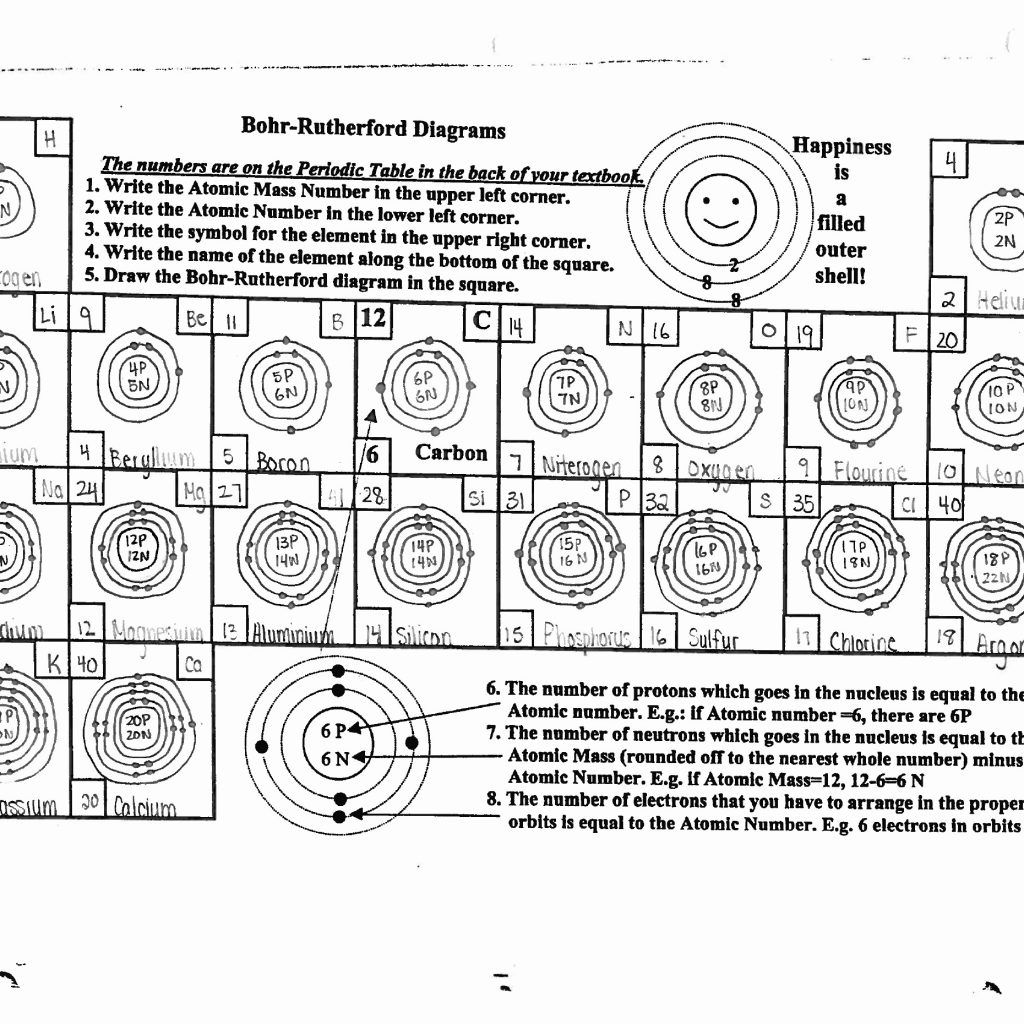
Before you can draw a Bohr model, you need to know which element you’re dealing with:
- Look at the atomic number, which tells you the number of protons (and for neutral atoms, also the number of electrons).
- Refer to the periodic table to find the element.
| Element | Atomic Number | Electron Configuration |
|---|---|---|
| Hydrogen | 1 | 1s1 |
| Helium | 2 | 1s2 |
| Lithium | 3 | 1s2 2s1 |

This table provides a quick reference for filling out electron shells:
3. Draw the Nucleus and Shells

Begin with the nucleus at the center:
- Draw a circle or ellipse to represent the nucleus.
- Label it with the number of protons and neutrons (mass number - atomic number).
- Draw concentric circles around the nucleus to represent electron shells.
💡 Note: For complex elements, you might not need to draw all shells; focus on the relevant ones for understanding electron behavior.
4. Place Electrons in Shells

Each shell can hold a specific number of electrons. Here’s how to place them:
- Start with the innermost shell (K shell) and fill it with 2 electrons.
- Move outward, filling each shell up to the maximum it can hold before adding electrons to the next shell.
- For atoms with many electrons, you might only need to draw the valence shell (outermost shell) for simplicity.
Example: Carbon

- 6 electrons in total.
- First shell: 2 electrons.
- Second shell: 4 electrons.
5. Application and Practice

To truly master Bohr models, you need:
- Repetitive Practice: Engage with numerous worksheets that present various elements.
- Problem Solving: Work through exercises that require you to calculate electron configurations, atomic numbers, and predict element identities.
- Conceptual Understanding: Relate Bohr models to periodic trends, chemical properties, and electron reactivity.
As you practice:
- Use different colored pens or markers to differentiate between shells or electron energy levels for a clearer visualization.
- Challenge yourself by predicting the chemical behavior of elements based on their Bohr model configurations.
The journey through the Bohr models worksheet is not merely an academic exercise; it's an exploration into the essence of matter. By understanding how electrons are arranged around the nucleus, you're not just learning a model but gaining insights into the fundamental rules that govern our chemical world.
What is the significance of the Bohr model in chemistry?

+
The Bohr model introduced a revolutionary way to visualize electron arrangement, providing a foundation for quantum mechanics and understanding atomic structure, which is critical for explaining chemical bonding and reactivity.
Can the Bohr model accurately depict all elements?

+
The Bohr model is a simplification that works well for lighter elements. For heavier elements or those with complex electron configurations, more advanced models like the quantum mechanical model are needed.
How does understanding electron configuration help in chemistry?

+
Understanding electron configuration allows you to predict chemical properties, behavior during reactions, and to classify elements into groups with similar properties.
What are the limitations of the Bohr model?

+
Limitations include its inability to explain multi-electron atoms accurately, the concept of fixed orbits which quantum mechanics disproves, and the absence of explaining chemical bonding or the atomic spectra of larger atoms.