Atomic Structure Worksheet: Answer Key Revealed
In the intricate world of chemistry, understanding the fundamentals of atomic structure is crucial. It's not just about knowing what an atom is but delving into its subatomic particles, electron configurations, and the way these components define the chemical properties of elements. This blog post will provide an in-depth exploration of atomic structure through a comprehensive worksheet answer key, offering insights and clarifications that students and enthusiasts alike can benefit from.
What is an Atom?

An atom is the basic building block of matter and consists of three types of particles:
- Protons: Positively charged particles found in the nucleus, contributing to the atomic mass and atomic number.
- Electrons: Negatively charged particles that orbit the nucleus and are involved in chemical reactions.
- Neutrons: Neutral particles in the nucleus that add to the atomic mass but not to the atomic number.

Atomic Structure and Numbering

The atomic number defines an element, which is the number of protons in the nucleus. Here are some key points:
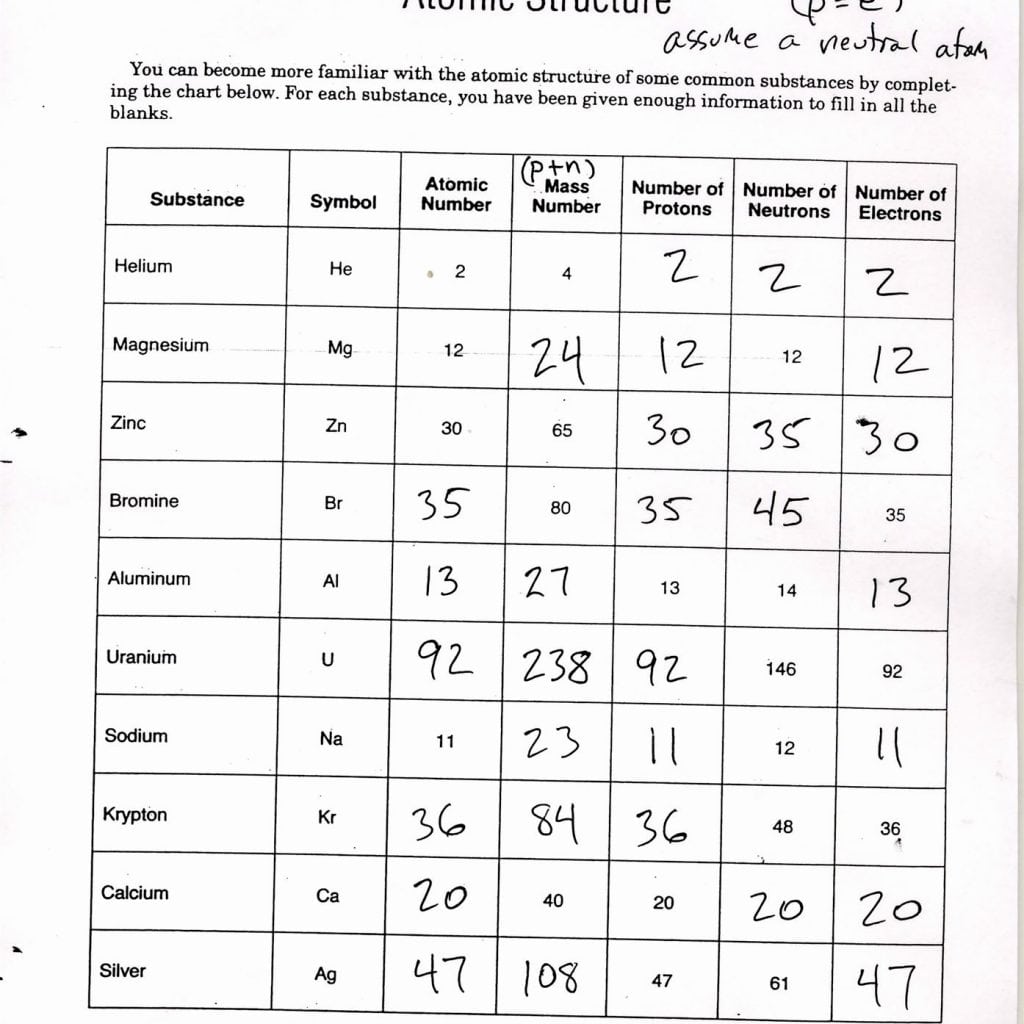
- Atomic Number - The number of protons, which also equals the number of electrons in a neutral atom.
- Mass Number - The sum of protons and neutrons in the nucleus.
| Element | Symbol | Atomic Number | Mass Number | Number of Protons | Number of Neutrons | Number of Electrons |
|---|---|---|---|---|---|---|
| Hydrogen | H | 1 | 1 | 1 | 0 | 1 |
| Helium | He | 2 | 4 | 2 | 2 | 2 |
| Oxygen | O | 8 | 16 | 8 | 8 | 8 |

⚠️ Note: In a neutral atom, the number of electrons equals the number of protons, which is the atomic number. However, atoms can gain or lose electrons, affecting their charge but not their identity as a specific element.
Electron Configuration

Electron configuration describes how electrons are distributed among the various atomic orbitals. Here's how to approach it:
- 1s, 2s, 2p, etc: The 's' and 'p' represent the type of orbital, while the number signifies the energy level.
- Each orbital can hold a maximum number of electrons (s holds 2, p holds 6, d holds 10, and f holds 14).
- The Aufbau principle, Pauli exclusion principle, and Hund's rule guide electron filling.
Example: Electron Configuration of Oxygen

Oxygen has an atomic number of 8, so it has 8 electrons. Here is the configuration:
- 1s2 (2 electrons in the 1s orbital)
- 2s2 (2 electrons in the 2s orbital)
- 2p4 (4 electrons in the 2p orbitals)
Ions and Isotopes

Atoms can gain or lose electrons to become ions, changing their charge:
- Cation: An atom loses electrons, resulting in a positive charge (e.g., Na+).
- Anion: An atom gains electrons, resulting in a negative charge (e.g., Cl-).
Isotopes are atoms of the same element with different numbers of neutrons, hence different mass numbers:
- Isotopes: Same atomic number but different mass numbers due to variations in neutron count.
Example: Isotopes of Hydrogen

Hydrogen has three isotopes:
- Hydrogen-1 (protium) has 1 proton and no neutrons.
- Hydrogen-2 (deuterium) has 1 proton and 1 neutron.
- Hydrogen-3 (tritium) has 1 proton and 2 neutrons.
💡 Note: Despite their neutron differences, isotopes have the same atomic number, which means they are the same element but with different physical properties like density or mass.
Application in Chemistry

Understanding atomic structure has far-reaching implications in chemistry:
- Chemical Bonding: Electrons' arrangement and availability lead to different types of bonds like ionic, covalent, and metallic.
- Periodic Table: Groups elements based on their atomic structure, especially electron configuration.
- Spectroscopy: Used to analyze the interaction of light with matter, revealing the electronic structure of atoms.
The atom's composition directly influences its behavior in chemical reactions. For instance, the number of electrons in the outermost shell (valence shell) determines an atom's reactivity and its ability to form bonds:
- An atom with a full valence shell is stable and typically inert (e.g., noble gases).
- Atoms with fewer than eight valence electrons tend to form bonds to achieve stability.
🌟 Note: Electron configuration and atomic structure are key to understanding why certain elements react with others, forming compounds with specific properties.
To wrap up, exploring atomic structure through a worksheet not only reinforces core knowledge but also highlights how foundational chemistry is in understanding the natural world. From the structure of an atom to the implications of electrons in bonding, everything around us can be explained through the basic building blocks of matter. This journey into the microscopic realm not only clarifies concepts like atomic numbers and isotopes but also connects these fundamental ideas to larger themes in chemistry, enhancing our appreciation of the subject.
What is the significance of the atomic number?

+
The atomic number uniquely identifies an element by defining the number of protons in its nucleus. This determines the element’s position in the periodic table and its chemical behavior.
How do isotopes affect an element’s properties?

+
Isotopes have the same chemical properties but different physical properties due to their varying mass. This can influence the stability, reactivity, and half-life in radioactive isotopes, affecting uses in medicine and industry.
Why are electron configurations important in chemistry?

+
Electron configurations show how electrons are arranged in an atom, influencing the atom’s reactivity, bonding, and overall chemical behavior. They help predict how elements will bond with others to form compounds.
Can an atom exist without electrons?

+
Yes, but only momentarily. An atom stripped of all its electrons becomes a highly unstable, positively charged ion (cation). It will immediately attempt to gain electrons to become stable.