5 Essential Tips for Understanding Acids, Bases, and Salts

Have you ever considered what really happens when you squeeze that lemon into your glass of water or when you scrub a stubborn stain with baking soda? These everyday actions are fundamentally rooted in chemistry, specifically in the behavior of acids, bases, and salts. Understanding these compounds can be a game-changer for both students and curious minds alike. Let's dive into the fascinating world of acids, bases, and salts and uncover why they play such critical roles in our daily lives.
1. The Basics of Acids

Acids are substances that donate protons (H⁺ ions) or accept electron pairs in chemical reactions. Here's what you need to know:
- pH Scale: The pH scale measures acidity or alkalinity from 0 to 14. A pH of 7 is neutral, below 7 is acidic, and above 7 is alkaline.
- Properties: Acids often taste sour, react with metals to form hydrogen gas, and turn blue litmus paper red.
- Examples: Lemon juice, vinegar, stomach acid, and citric acid.
- Strength: The strength of an acid depends on its ability to ionize or dissociate in water. Strong acids like hydrochloric acid (HCl) fully ionize, while weak acids like acetic acid (vinegar) only partially ionize.
2. Understanding Bases

Bases, on the other hand, accept protons or donate an electron pair:
- pH Scale: As mentioned, bases have a pH greater than 7.
- Properties: They feel slippery, turn red litmus paper blue, and can neutralize acids.
- Examples: Soap, ammonia, baking soda, and lye (sodium hydroxide).
- Strength: Like acids, the strength of bases depends on their ionization in water. Strong bases like NaOH fully ionize, while weak bases like ammonia partially ionize.
3. The Role of Salts
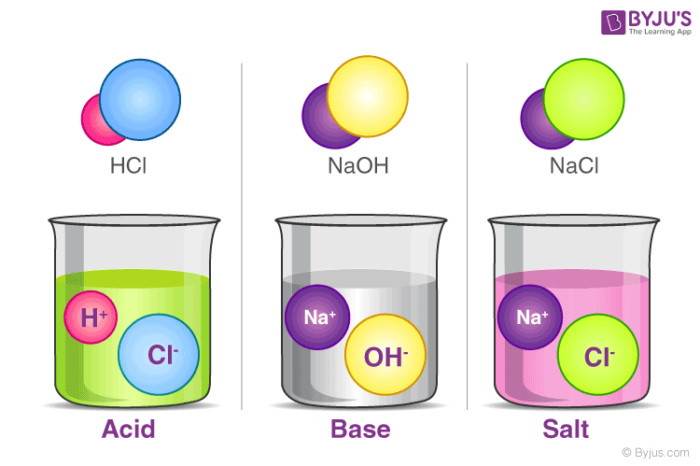
Salts are formed when acids react with bases, producing a compound that neither has acid nor base properties:
- Formation: The general reaction is: acid + base = salt + water.
- Neutralization: This reaction leads to a neutral solution (pH = 7), but exceptions exist where salts can still impart pH changes.
- Properties: Salts are typically crystalline solids with high melting points, and they conduct electricity when dissolved in water or melted.
- Common Salts: Sodium chloride (table salt), calcium carbonate (chalk), and potassium nitrate (used in fertilizers).
4. Acid-Base Reactions

Understanding how acids and bases interact is crucial:
- Neutralization: This is the most common acid-base reaction, where acids react with bases to form water and a salt, thus neutralizing each other's properties.
- Buffer Systems: These are mixtures of a weak acid and its conjugate base, or a weak base and its conjugate acid, which resist pH changes when small amounts of acids or bases are added.
- Indicators: These are substances that change color at specific pH levels, helping us determine whether a solution is acidic or basic.
5. Practical Applications in Everyday Life

The chemistry of acids, bases, and salts isn't just for textbooks; they're all around us:
- Cleaning: Baking soda and vinegar (sodium bicarbonate and acetic acid) combine to fizz and clean due to their acid-base reaction.
- Medicine: Stomach antacids use weak bases like magnesium hydroxide to neutralize excess stomach acid.
- Agriculture: Soil pH is adjusted by adding acids or bases to optimize plant growth conditions.
- Food: Citric and ascorbic acids (vitamin C) are used in food preservation, while bases like sodium bicarbonate are used as leavening agents in baking.
- Environmental Impact: Acid rain, caused by pollutants like sulfur dioxide, has a detrimental effect on the environment by lowering soil and water pH, which can be combated by liming to raise pH levels.
In closing, the intricate dance of acids, bases, and salts is not only a fundamental aspect of chemistry but also a cornerstone of our daily existence. From the microlevel of our digestive systems to the macrolevel of industrial processes and environmental management, these compounds shape the world around us. By understanding their properties and reactions, we can better manage our environment, optimize health, and harness chemical reactions for practical applications. This knowledge opens the door to a deeper appreciation of science and its tangible impact on our lives.
What is pH and why is it important?
+
The pH scale measures the acidity or alkalinity of a substance, ranging from 0 to 14. It’s crucial in biology, chemistry, and environmental science because the pH level affects enzyme function, biological processes, and the health of ecosystems. For instance, the human body maintains a blood pH close to 7.4; any significant deviation can be life-threatening.
Can acid-base reactions be used in energy storage?

+
Yes, acid-base reactions are involved in electrochemical cells like batteries. For example, lead-acid batteries use sulfuric acid in their reaction to generate electrical energy. The potential for energy storage also lies in pH-dependent reactions that can store and release energy through changes in chemical potential.
How does soil pH affect plant growth?

+
Soil pH influences the availability of nutrients to plants. If the pH is too high or too low, essential nutrients like phosphorus, iron, and manganese become less available, which can inhibit plant growth. Farmers often adjust soil pH by adding lime (to raise pH) or sulfur (to lower pH) to optimize the conditions for specific crops.