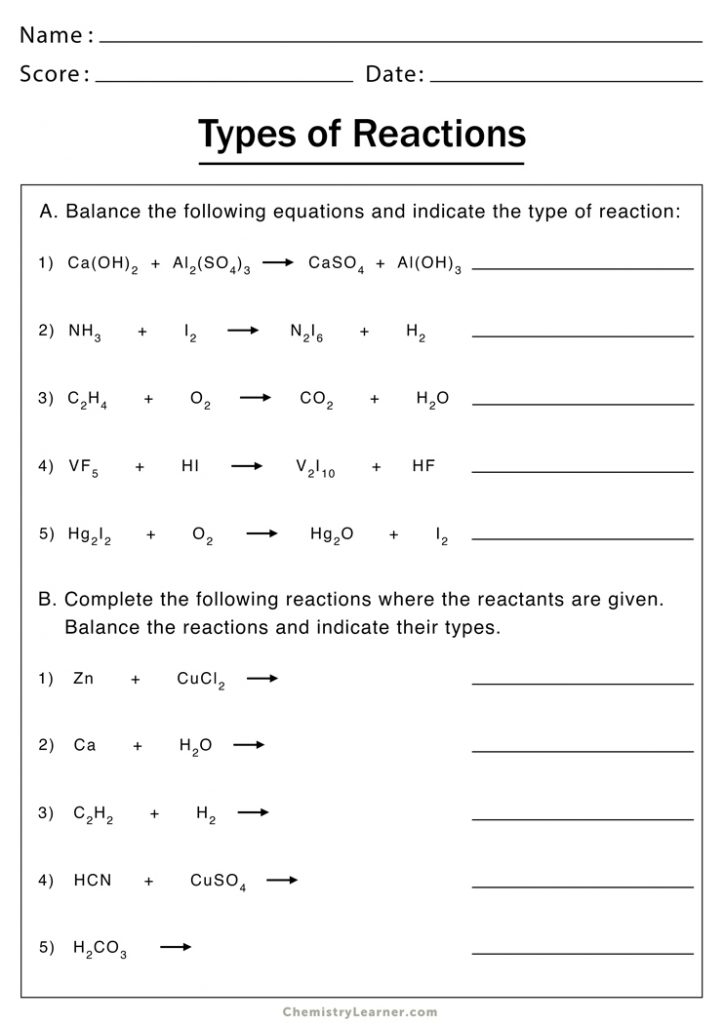
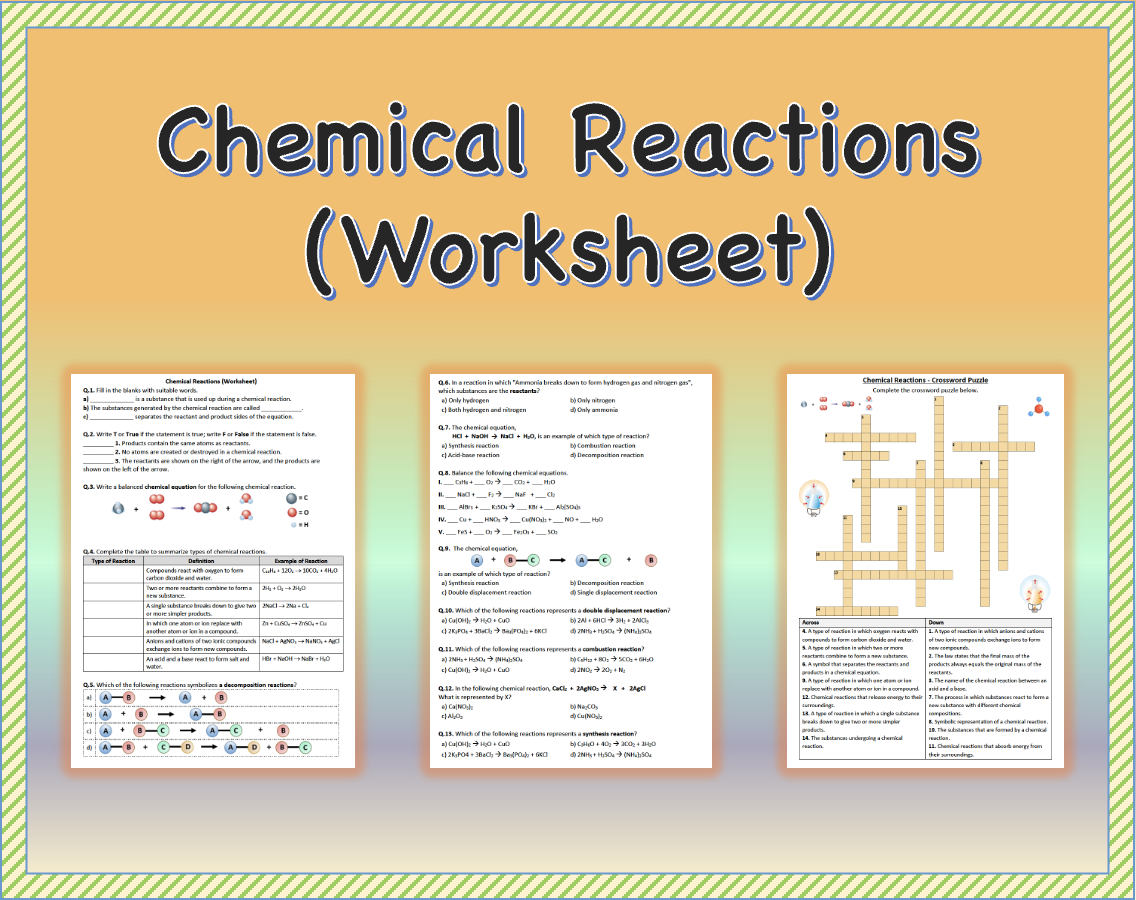
5 Key Strategies for Mastering Synthesis Reactions Worksheet 2

Understanding synthesis reactions, also known as combination reactions, is pivotal for students in chemistry. These reactions involve two or more substances combining to form a single compound. In this post, we'll delve into the top 5 strategies to master synthesis reactions, particularly when working through a synthesis reactions worksheet. This will not only enhance your grasp of chemical synthesis but also equip you with practical tips to excel in your chemistry studies.
Strategy 1: Understand the Basics

Mastering synthesis reactions begins with a solid understanding of the fundamental principles. Here's what you should focus on:
- Definition: A synthesis reaction involves combining at least two substances to produce a single compound. The general equation can be written as A + B → AB.
- Types of synthesis reactions:
- Combination of elements: e.g., 2Na + Cl2 → 2NaCl
- Combination with oxides: e.g., C + O2 → CO2
- Formation of acids and bases: e.g., SO3 + H2O → H2SO4
- Common misconceptions:
- Synthesis reactions do not always involve an element and a compound; both can be elements or compounds.
- They are not only confined to ionic compounds; covalent compounds also undergo synthesis reactions.
🧪 Note: A thorough understanding of chemical formulas and balanced equations is crucial. Revisit these concepts if needed.
Strategy 2: Recognize Patterns in Chemical Equations
Synthesis reactions often follow recognizable patterns, making them easier to identify and solve:
- Element-Element Synthesis: When two elements combine, they typically form a compound with a 1:1 ratio, adjusting for electronegativity.
- Oxide Synthesis: When an element or compound combines with oxygen, the resulting compound is often an oxide.
- Water as a Reagent: Adding water to certain compounds (like SO3) leads to the formation of acids or bases.
- Charge Balancing: In ionic synthesis reactions, ensure the total charge on either side of the equation balances out.
By recognizing these patterns, you can quickly categorize and solve synthesis reactions during your worksheet exercises.
Strategy 3: Practice Balanced Equations

The heart of mastering synthesis reactions lies in balancing chemical equations. Here are steps to improve your balancing skills:
- Identify Reactants and Products: Clearly distinguish between reactants and products in the equation.
- Use Mole Ratios: Start by setting up simple ratios based on the simplest ion or molecule formulas.
- Work Left to Right: Balance the equation from left to right, starting with the most complex compound or ion.
- Check for Fractional Coefficients: If you encounter fractions, multiply all coefficients by the smallest common denominator.
- Double-Check: After balancing, recheck your equation to ensure that atoms on both sides match in number and type.
| Example | Balancing Process |
|---|---|
| 2Na + Cl2 → 2NaCl | 1. Sodium is balanced with a coefficient of 2 before NaCl.
2. Chlorine, being diatomic, also requires a coefficient of 2. 3. Ensure both sides match: 2Na, 2Cl → 2Na, 2Cl. |
🧪 Note: Balancing equations is not just about numbers; it's about ensuring conservation of mass and charge.
Strategy 4: Use Mnemonics and Tricks

To make synthesis reactions more memorable, consider these mnemonic devices:
- Remembering Synthesis Types: Use the phrase "Single Set, Dual Bond" to recall that synthesis generally involves elements or compounds forming one product.
- Balancing Charge: For ionic synthesis, think "POSH" - Positive Oxidants Should Have the correct charge balance.
- Recognizing Reactants: If you see an element and an oxide, think "Oxydus" (from Oxide), which can help remind you that the product might be an oxide.
These mnemonics can simplify the process of solving synthesis reactions during your study sessions.
Strategy 5: Apply Active Learning Techniques

Active learning significantly boosts understanding and retention in chemistry. Here are some techniques to employ:
- Flashcards: Create flashcards with synthesis reactions on one side and the balanced equations on the other.
- Concept Mapping: Develop concept maps linking synthesis reactions to their types, reactants, products, and balancing techniques.
- Group Study: Engage with peers to explain reactions to each other, which reinforces understanding.
- Simulations and Virtual Labs: Use online chemistry simulations to visualize synthesis reactions in action.
Active learning not only helps in mastering synthesis reactions but also makes learning chemistry more interactive and engaging.
🧪 Note: Regular practice and application of these techniques will enhance your proficiency with synthesis reactions.
As we've explored these strategies, keep in mind that mastering synthesis reactions involves a blend of understanding, pattern recognition, balancing, mnemonic aids, and active learning. By implementing these five key strategies, you can confidently tackle any synthesis reactions worksheet, deepening your grasp of this fundamental chemical concept. Remember, chemistry is not just about memorization but understanding the underlying principles that govern reactions, which in turn allows you to predict and solve new chemical problems with ease.
What are the most common mistakes students make in synthesis reactions?

+
Common mistakes include not balancing the equation correctly, neglecting the states of matter, and confusing synthesis with other reaction types like decomposition or displacement.
How can I differentiate synthesis reactions from other types of reactions?

+
Synthesis reactions combine two or more substances into one. Look for a single product on the right side of the equation, as opposed to decomposition (breaking down into multiple products) or displacement reactions.
Can all elements or compounds participate in synthesis reactions?

+
Generally, yes, but some elements or compounds might not react under normal conditions or might need special conditions (like high temperature or pressure) to combine. Understanding reactivity series and electronegativity helps in predicting which reactions are likely to occur.