Equilibrium Expressions & Calculations: Worksheet Answers Explained

Chemical equilibrium is a fascinating topic within chemistry, where reactions reach a state of balance, and the rate of the forward reaction equals that of the reverse reaction. This equilibrium allows for reactions that can seem to halt, but in reality, they continue at equal and opposite rates. This blog post delves deep into the heart of equilibrium expressions and calculations, offering an in-depth look at worksheets and answers to help both students and enthusiasts better understand this core principle.
Understanding Equilibrium

Chemical equilibrium is defined when the concentrations of reactants and products no longer change over time. Here's a brief rundown:
- Dynamic Equilibrium: At this point, reactions don't stop; they just balance out where the rate of product formation equals the rate of product decomposition back into reactants.
- Static Equilibrium: No movement at all, which is less common in chemical reactions. Here, the concentrations of all species remain constant because the rates of forward and reverse reactions are equal.
⏰ Note: Remember, equilibrium doesn't mean the reaction has stopped but rather it has balanced out.
Equilibrium Expressions

The equilibrium constant, K, expresses the ratio of the concentration of products to the concentration of reactants at equilibrium. Here's how it's calculated:
Writing Equilibrium Expressions
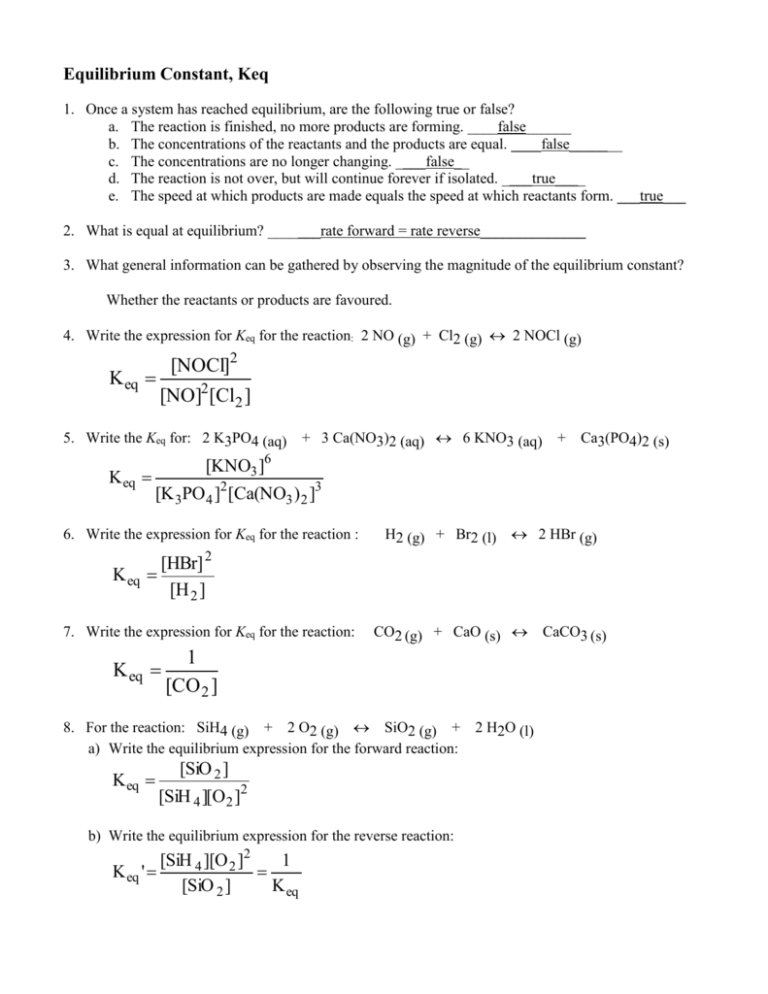
To write the equilibrium expression:
- Write out the balanced chemical equation.
- Express Keq (equilibrium constant) as the ratio of products to reactants, with each raised to the power of their stoichiometric coefficients.
| Equation | Equilibrium Expression (Keq) |
|---|---|
| aA + bB ⇌ cC + dD | Keq = [C]c[D]d / [A]a[B]b |

✍️ Note: Pure solids and liquids are not included in the equilibrium expression because their concentrations do not change significantly.
Calculating Equilibrium Concentrations

With Keq in hand, one can predict the concentrations at equilibrium using the following approach:
ICE Tables

Initial Change Equilibrium (ICE) tables help visualize changes in concentration:
- Initial: List the initial concentrations.
- Change: Determine how much the concentrations change based on stoichiometry.
- Equilibrium: Calculate the concentrations at equilibrium.
Example Calculation
Consider the reaction:
H2(g) + I2(g) ⇌ 2HI(g)
If the initial concentrations of H2 and I2 are both 0.5 M and Keq is 50 at 448°C, we can:
- Set up the ICE table:
| Species | Initial (M) | Change (M) | Equilibrium (M) |
|---|---|---|---|
| H2 | 0.5 | -x | 0.5-x |
| I2 | 0.5 | -x | 0.5-x |
| 2HI | 0 | +2x | 2x |
Solving for x using Keq gives us:
Keq = (2x)2 / ((0.5-x)(0.5-x)) = 50
Through algebra or calculation, we find x = 0.4 M, leading to:
- [H2] = [I2] = 0.1 M
- [HI] = 0.8 M
🧮 Note: For reactions with high Keq, like this one, equilibrium heavily favors the products.
Applications and Extensions

Equilibrium calculations are not just academic exercises; they have practical applications:
- Reaction Optimization: Understand how changing conditions (temperature, pressure, concentration) affects equilibrium to optimize industrial reactions.
- Drug Design: Equilibrium principles are vital in understanding how drugs bind to receptors, influencing their potency and duration of action.
- Environmental Chemistry: Predict the fate of pollutants and their impact on the environment through equilibrium considerations.
Le Chatelier's Principle

Le Chatelier's principle helps predict how a system at equilibrium responds to changes in conditions:
- If a change is applied, the system adjusts to counteract the change.
- Temperature: Exothermic reactions shift left with increased temperature; endothermic reactions shift right.
- Pressure: Increasing pressure shifts equilibrium towards fewer moles of gas.
- Concentration: Adding reactants pushes the reaction towards products and vice versa.
Equilibrium in Real Life

Equilibrium isn't just theoretical; it's all around us:
- The concentration of blood glucose is regulated by a biological equilibrium.
- When we reach equilibrium, the atmosphere's composition helps stabilize the climate.
- In electronics, the balance of electrical charges in circuits can be considered a form of equilibrium.
Equilibrium in chemistry, though intricate, illuminates how nature maintains balance. Here are some key takeaways:
- Chemical reactions reach equilibrium when the rate of forward reaction equals that of reverse reaction.
- The equilibrium constant, K, is a quantitative way to describe the equilibrium state.
- ICE tables are invaluable tools for calculating equilibrium concentrations.
- Le Chatelier's principle is key to understanding how systems adjust to changes in conditions.
Understanding equilibrium allows us not just to predict but also to manipulate reaction conditions to achieve desired outcomes. This control is pivotal in fields ranging from pharmaceuticals to environmental science, showcasing the real-world importance of these principles.
What is chemical equilibrium?

+
Chemical equilibrium is a state in a reversible reaction where the rate of the forward reaction equals the rate of the reverse reaction, and the concentration of reactants and products remain constant over time.
Why are pure solids and liquids not included in the equilibrium expression?

+
Their concentrations do not significantly change during the reaction, making them effectively constants, which get incorporated into the equilibrium constant K.
Can equilibrium be affected by catalysts?

+
Catalysts speed up both forward and reverse reactions equally, not changing the equilibrium position, only the time to reach it.