5 Key Facts About Isotopes You Should Know
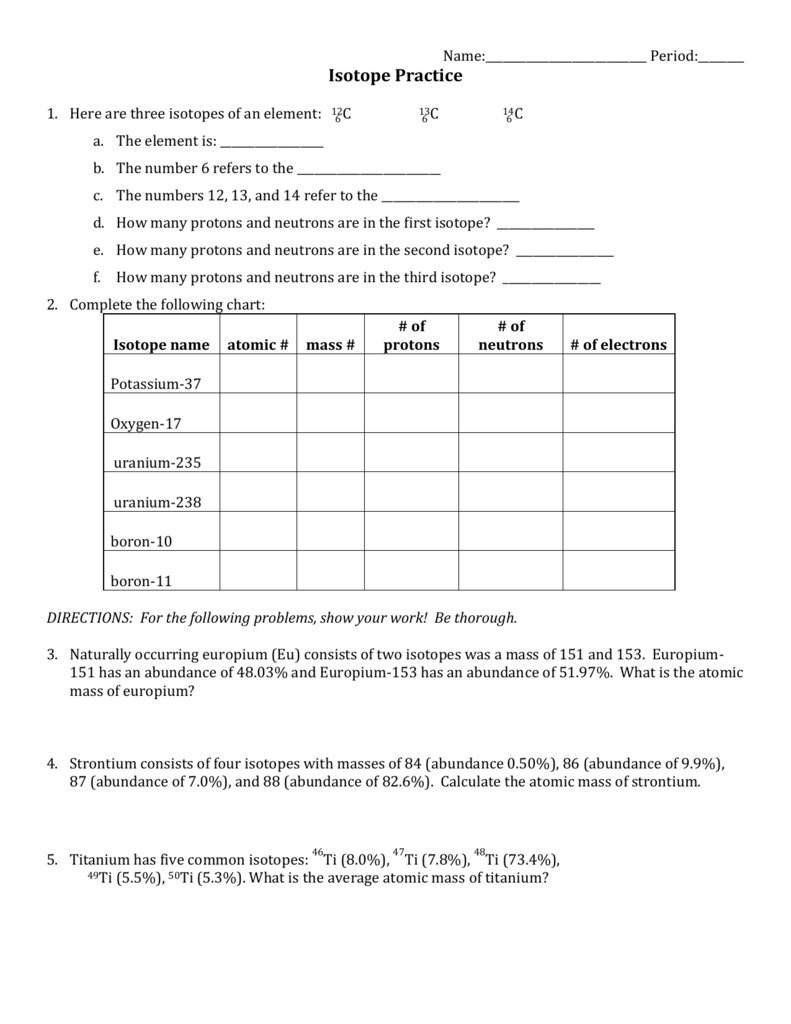
Isotopes are fascinating elements in the vast universe of chemistry and physics, playing crucial roles in science, industry, and even daily life. Whether you're a science enthusiast, a student, or just someone curious about the world, understanding isotopes can offer insights into how the elements around us work and why they matter. Here are five key facts about isotopes you should know:
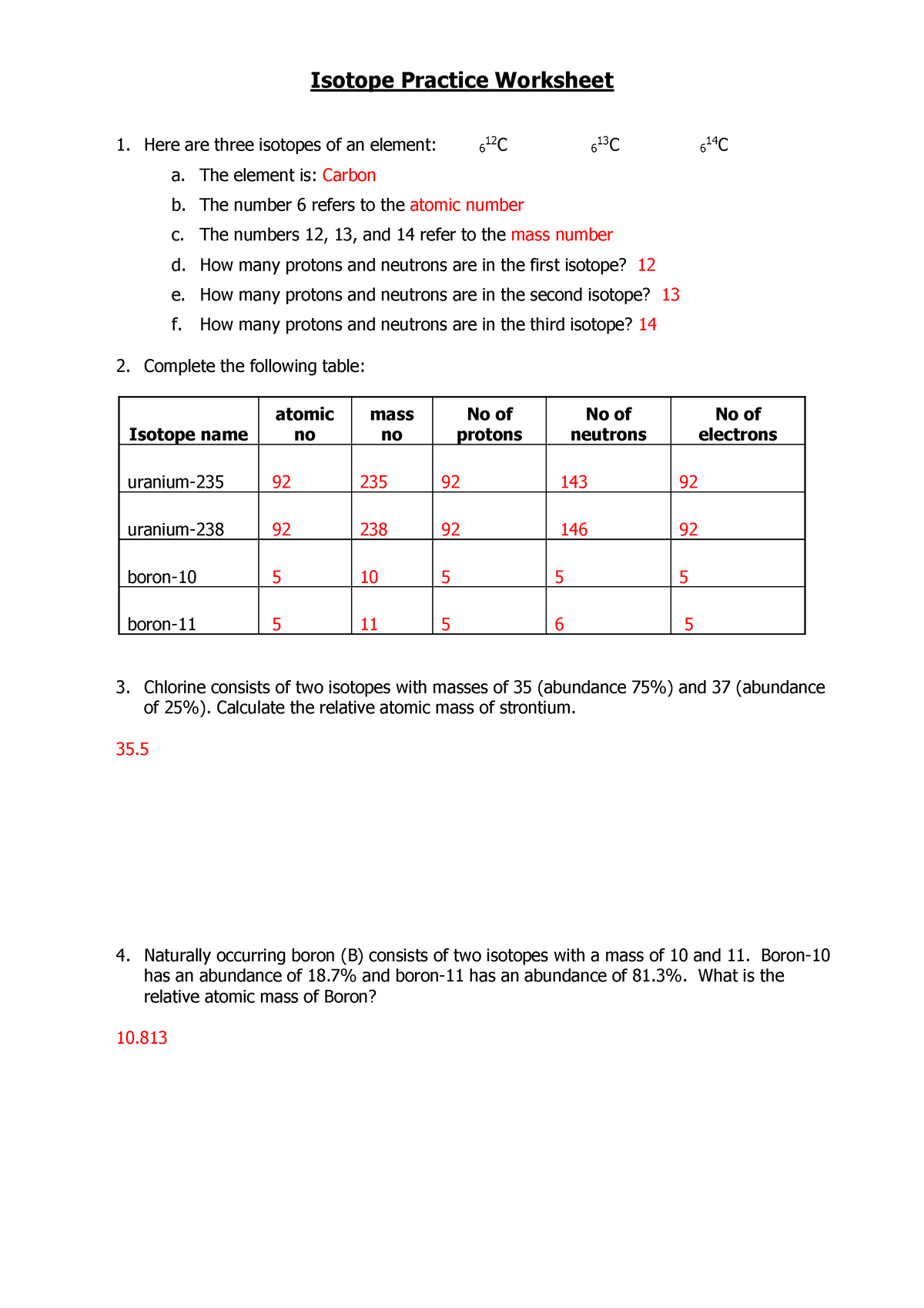
What Are Isotopes?
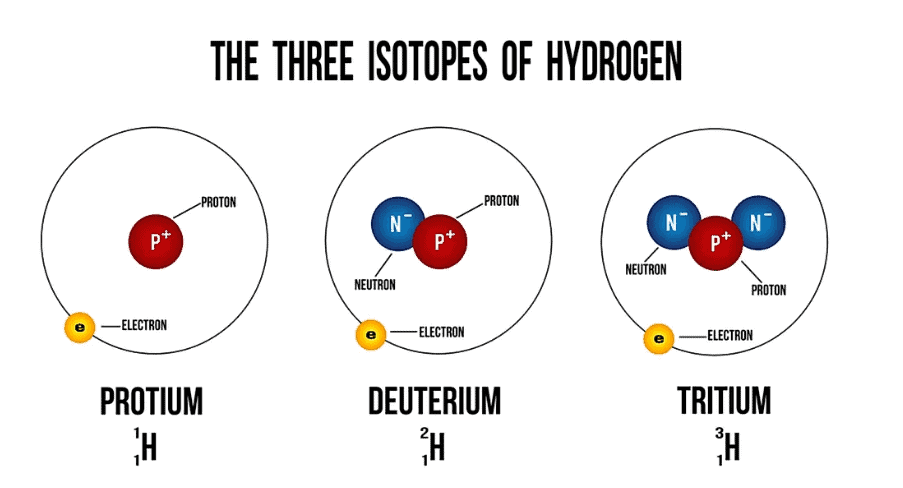
Isotopes are variants of a particular chemical element. These variants share the same number of protons, and hence the same atomic number, which defines the element. However, they differ in the number of neutrons. Here are the key points:
- Atomic Number: All isotopes of an element have the same atomic number.
- Mass Number: Each isotope has a different mass number because of the differing number of neutrons.
- Atomic Weight: The atomic weight of an element is typically the weighted average of the atomic weights of its naturally occurring isotopes.
💡 Note: The presence of isotopes explains why the atomic weights of elements in the periodic table are not whole numbers.
Isotope Notation
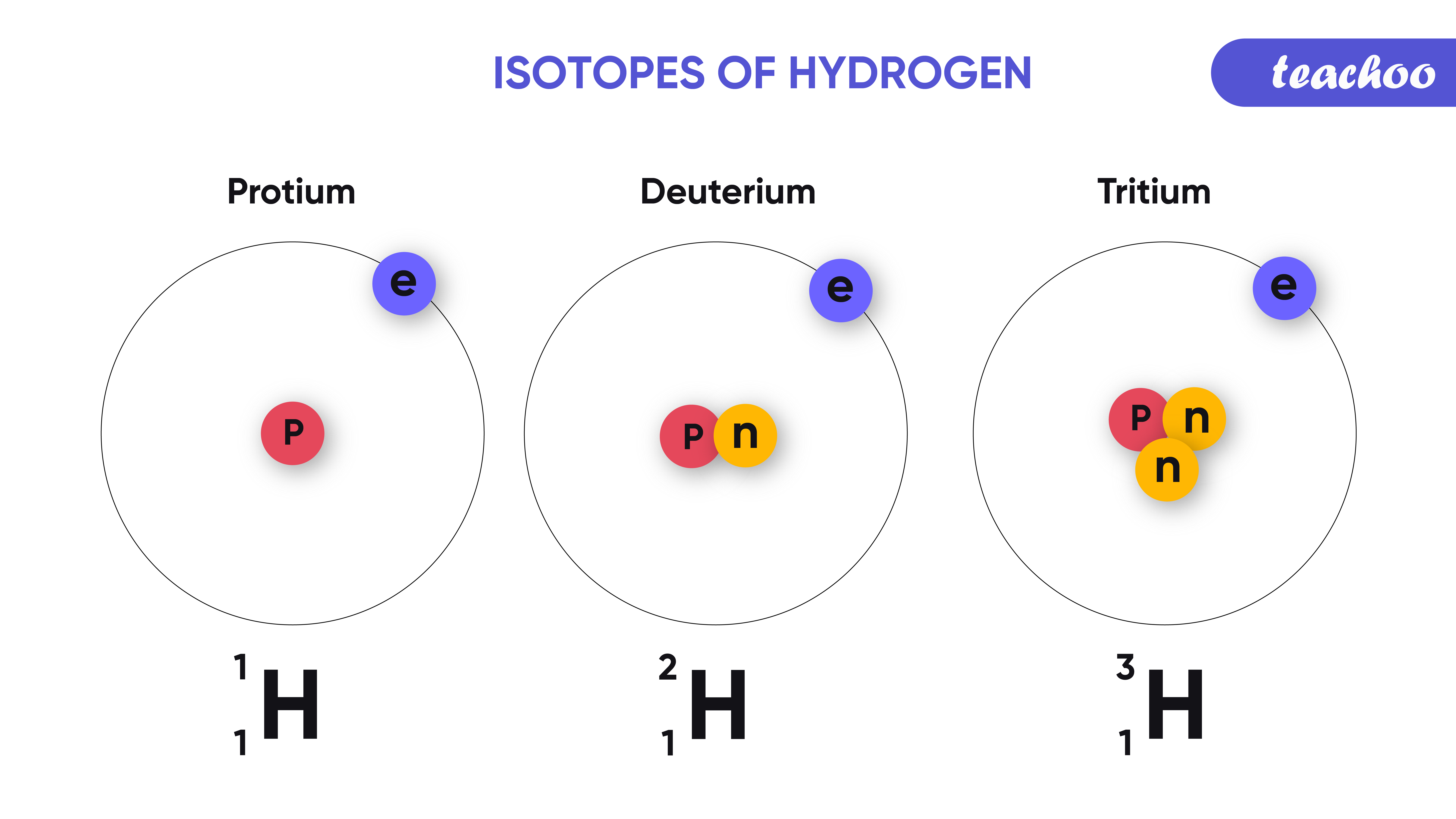
Isotopes can be represented in a specific notation. Here’s how:
- Element Symbol: The chemical symbol for the element.
- Atomic Number: Positioned in the subscript before the element symbol.
- Mass Number: Positioned in the superscript before the element symbol.
For example, the isotope of carbon with six protons and six neutrons would be represented as:
¹²C
| Element | Isotope | Number of Protons | Number of Neutrons |
|---|---|---|---|
| Carbon | ¹²C | 6 | 6 |
| Carbon | ¹³C | 6 | 7 |
| Carbon | ¹⁴C | 6 | 8 |
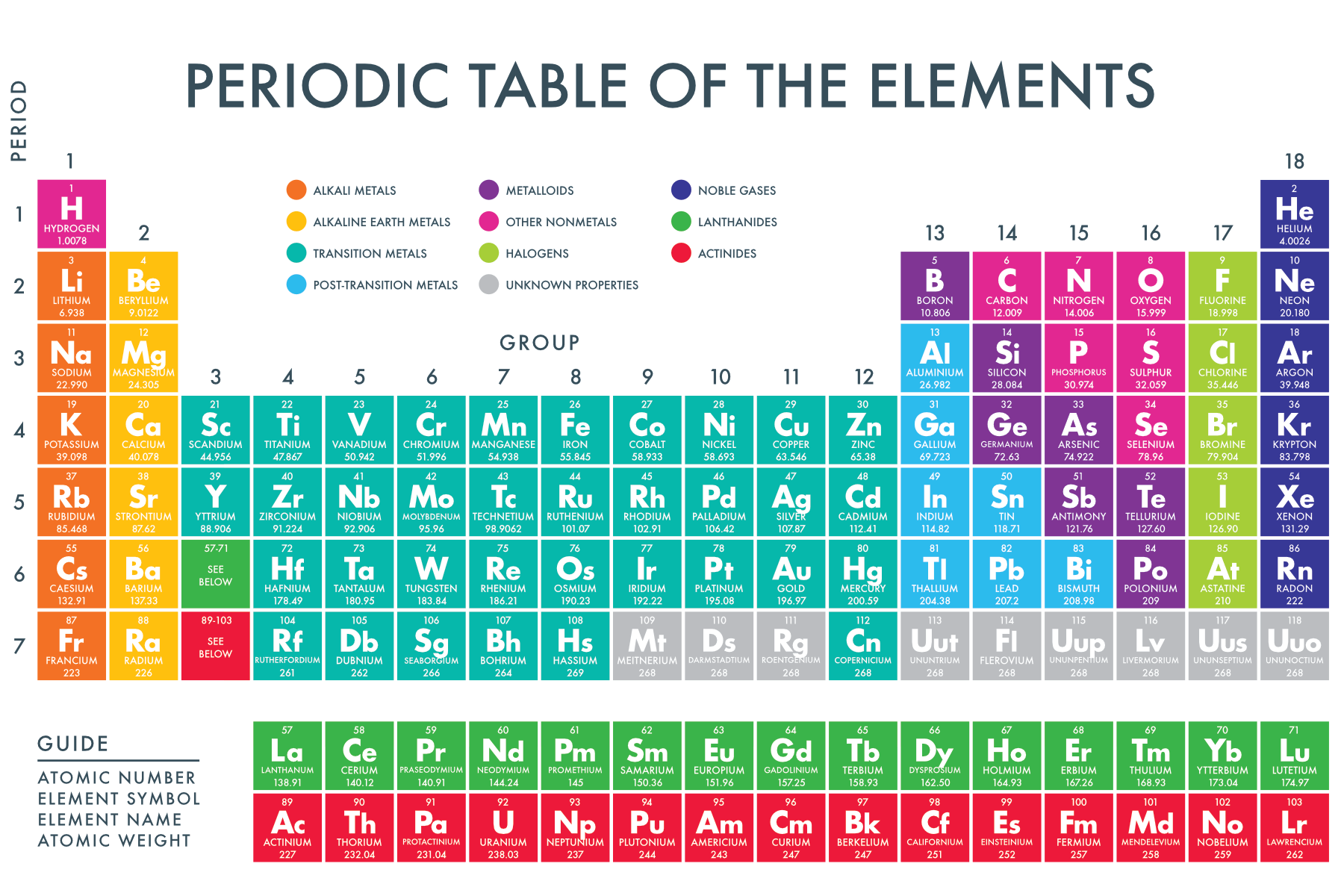
Types of Isotopes

Isotopes are categorized into two types:
- Stable Isotopes: These are isotopes that do not undergo radioactive decay. An example is Carbon-12.
- Unstable or Radioisotopes: These isotopes decay over time, emitting radiation. An example is Uranium-235.
🚫 Note: Not all elements have stable isotopes; many are known for their unstable, radioactive forms.
The Role of Isotopes in Our Lives

Isotopes have wide-ranging applications:
- Medicine: Radioactive isotopes are used in diagnostic procedures like PET scans and in treatments like radiotherapy for cancer.
- Industry: Isotopes are used in various industries for testing the thickness or integrity of materials.
- Archaeology: Carbon-14 dating helps estimate the age of archaeological artifacts.
- Nuclear Power: Uranium isotopes are fundamental in nuclear reactors for power generation.
Understanding isotopes helps in better using and harnessing their potential in these applications.
Isotopes and Environmental Science
Isotopes also play a significant role in environmental research:
- Pollution Tracking: Isotopes can trace pollutants back to their source, helping with environmental management.
- Climate Research: Ice core samples contain isotopes that provide insights into past climate conditions.
- Aquifer Management: Isotopic analysis helps in understanding groundwater movement and age.
The study of isotopes in the environment sheds light on processes not visible through other means.
Summing up, isotopes are more than just variations of elements; they are windows into the past, tools for the present, and harbingers of potential future technological advancements. Their differences in neutron counts lead to applications that range from understanding the age of our planet to saving lives through medical advancements. Isotopes are a fundamental part of our scientific understanding and daily life, impacting fields from medicine to environmental science. Their study enriches our knowledge of the natural world and our ability to manipulate it for the betterment of society.
What is the most common isotope of an element?

+
The most common isotope is usually the one with the highest natural abundance. For example, Carbon-12 is the most common isotope of carbon.
Can isotopes change into different elements?

+
Yes, through processes like radioactive decay or nuclear transmutation, an isotope can transform into another element if its nucleus changes by gaining or losing particles.
Why are isotopes important in medicine?
+
Isotopes like Technetium-99m are used in diagnostic imaging because they emit gamma rays that can be detected externally, allowing doctors to see inside the body without invasive surgery.
What is isotope dating?

+
Isotope dating, or radiometric dating, uses the decay of radioactive isotopes to estimate the age of rocks, fossils, and archaeological artifacts. Carbon-14 dating is the most well-known method.
Are isotopes stable or unstable?
+
Isotopes can be either stable or unstable. Stable isotopes do not undergo radioactive decay, while unstable isotopes, known as radioisotopes, decay over time.