5 Tips for Acing Your Vsepr Theory Worksheet

Mastering VSEPR Theory (Valence Shell Electron Pair Repulsion) can seem daunting at first, but with the right strategies, you can excel in understanding and applying this fundamental concept in chemistry. Whether you're a high school student or an undergrad, these tips will help you navigate through your VSEPR theory worksheet with confidence. Here's how you can ace your VSEPR assignments:
1. Understand the Basics

Before you start working on your VSEPR worksheet, make sure you have a solid grasp of the following:
- Electron Domains: These are regions of high electron density around an atom, including lone pairs, single bonds, double bonds, and triple bonds.
- Molecular Geometry: How the atoms within a molecule are positioned in 3D space.
- Electron Pair Repulsion: Electron pairs repel each other and will try to position themselves as far apart as possible.
🔍 Note: Always differentiate between the electron domain and molecular geometry as they can be different, especially when dealing with lone pairs.
2. Draw and Label Lewis Structures


Begin by drawing the Lewis structure for each molecule in your worksheet:
- Determine the total number of valence electrons for each atom.
- Calculate the formal charge to ensure the most plausible structure.
- Identify lone pairs and bonding pairs.
Lewis structures provide a visual representation, helping you to count electron domains and predict the shapes of molecules based on VSEPR.
3. Recognize Electron Domain Geometries
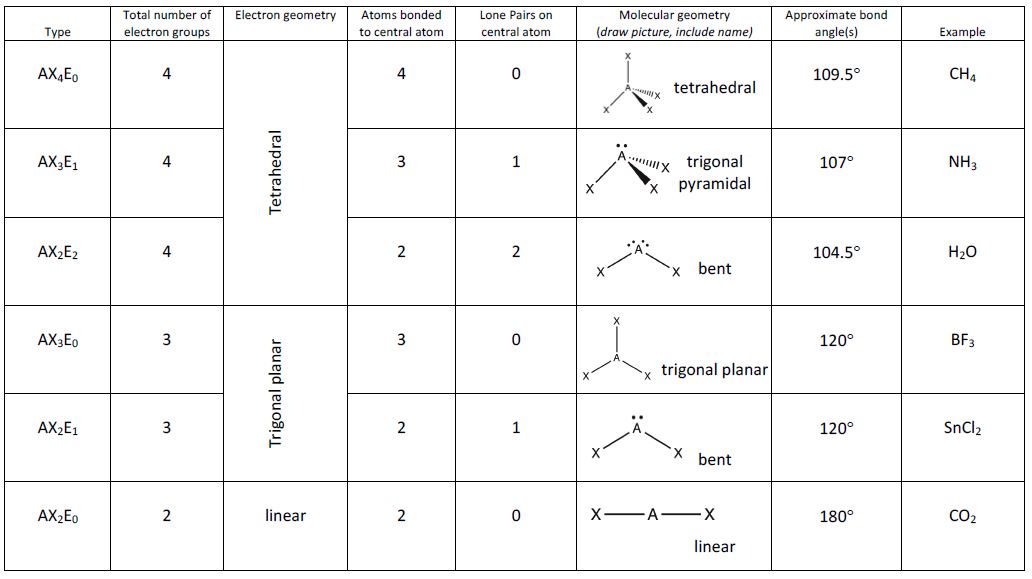
Once you have the Lewis structure, use the following steps:
- Count the number of electron domains around the central atom.
- Refer to VSEPR tables or diagrams to match the number of domains to the geometry:
| Number of Domains | Electron Domain Geometry |
|---|---|
| 2 | Linear |
| 3 | Trigonal Planar |
| 4 | Tetrahedral |
| 5 | Trigonal Bipyramidal |
| 6 | Octahedral |

Knowing these geometries will give you a base from which you can infer molecular geometry when lone pairs are present.
4. Account for Lone Pairs

VSEPR theory posits that lone pairs exert more repulsion than bonding pairs. Here’s how to account for them:
- Lone pairs repel more, altering the bond angles slightly.
- The molecular shape will often differ from the electron domain geometry due to lone pairs:
- A linear electron domain might yield a bent molecule.
- A tetrahedral domain could lead to trigonal pyramidal or bent structures.
Sketching the molecules with proper bond angles helps to visualize these effects.
5. Practice with Real Examples

Theory is great, but practice is key:
- Use everyday molecules like water (H2O), ammonia (NH3), methane (CH4), and phosphorus pentachloride (PCl5) for practice.
- Work through the steps for each molecule, predicting geometry and checking your work against known structures.
💡 Note: Don't just memorize shapes; understand why molecules take the shapes they do based on electron repulsion.
To wrap up, mastering VSEPR theory involves understanding the principles behind electron pair repulsion, accurately drawing Lewis structures, and applying electron domain geometries to deduce molecular shapes. Always remember to consider the effects of lone pairs on molecular geometry. Practice is your best friend here, turning theory into practical skills. With these strategies, you can approach your VSEPR worksheet with confidence, knowing you have the tools to visualize and understand the complex 3D structures of molecules.
What is the significance of the electron domain vs. the molecular geometry?
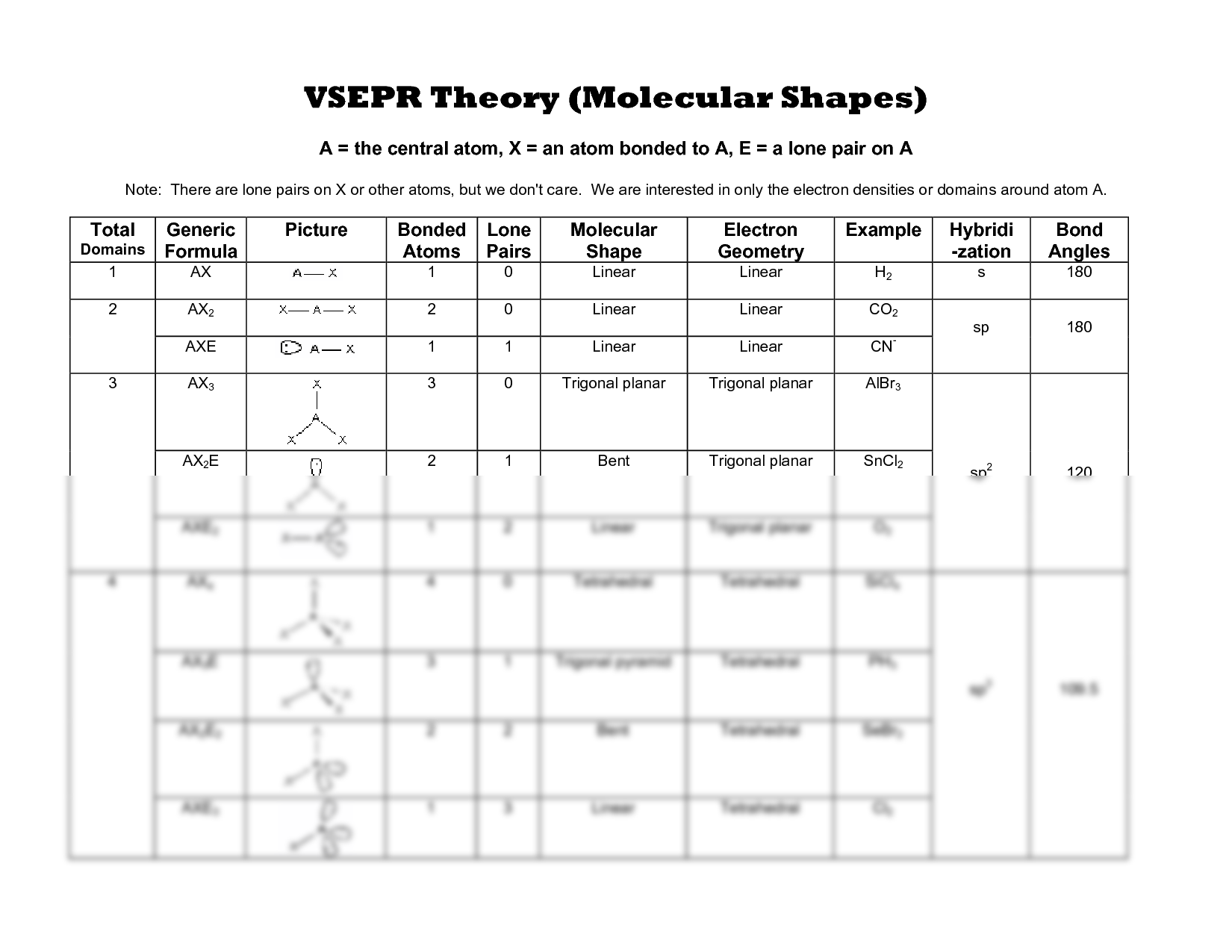
+
While electron domain geometry refers to the arrangement of all electron pairs around a central atom, molecular geometry only considers the arrangement of atoms, ignoring lone pairs. The difference accounts for the deviations in shape due to the greater repulsion of lone pairs.
Why do lone pairs exert more repulsion than bonding pairs?

+
Lone pairs occupy a greater volume around an atom than bonding pairs do since they are not shared. This additional space leads to a greater repulsive force, causing a slight increase in bond angles between atoms.
How can I easily identify the number of electron domains?

+
Count every electron region around the central atom. Single, double, and triple bonds each count as one domain, as do lone pairs.
Are there exceptions to VSEPR predictions?

+
Yes, some molecules like those involving transition metals, or where steric effects or resonance structures are significant, might not perfectly conform to VSEPR theory due to other chemical influences.
Why is it important to practice with real examples?

+
Practical examples solidify theoretical knowledge by providing tangible applications of VSEPR principles. This practice helps in recognizing patterns and understanding how electron pair repulsion affects molecular shape in real-world molecules.