Valence Electrons Worksheet Answers: Boost Your Chemistry Skills

Understanding valence electrons is fundamental in mastering chemistry, particularly when you're delving into the world of bonding, chemical reactions, and the periodic table. Let's explore this concept with a series of valence electrons worksheet answers to help you boost your chemistry skills and deepen your understanding of how atoms interact.
What Are Valence Electrons?
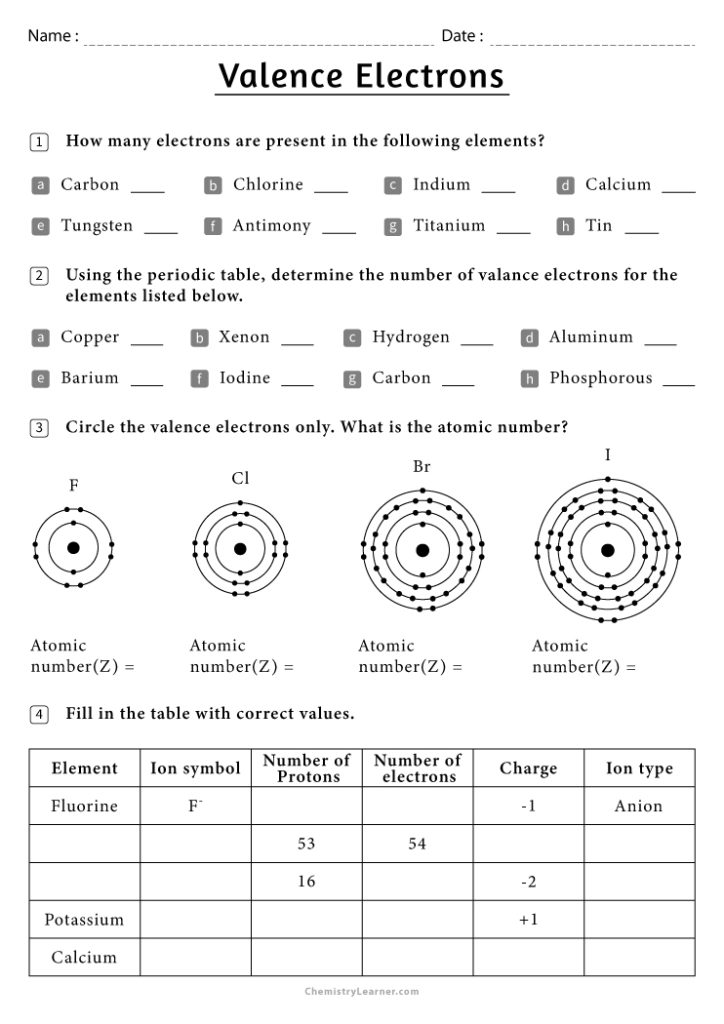
Valence electrons are the outermost electrons in an atom, which are involved in the bonding with other atoms. These electrons determine an element’s:
- Reactivity
- Type of chemical bonds it can form
- Chemical properties
They reside in the highest energy levels or shells and are crucial for understanding periodic trends like:
- Ionization energy
- Electronegativity
- Electron affinity
Valence Electron Worksheet Answers
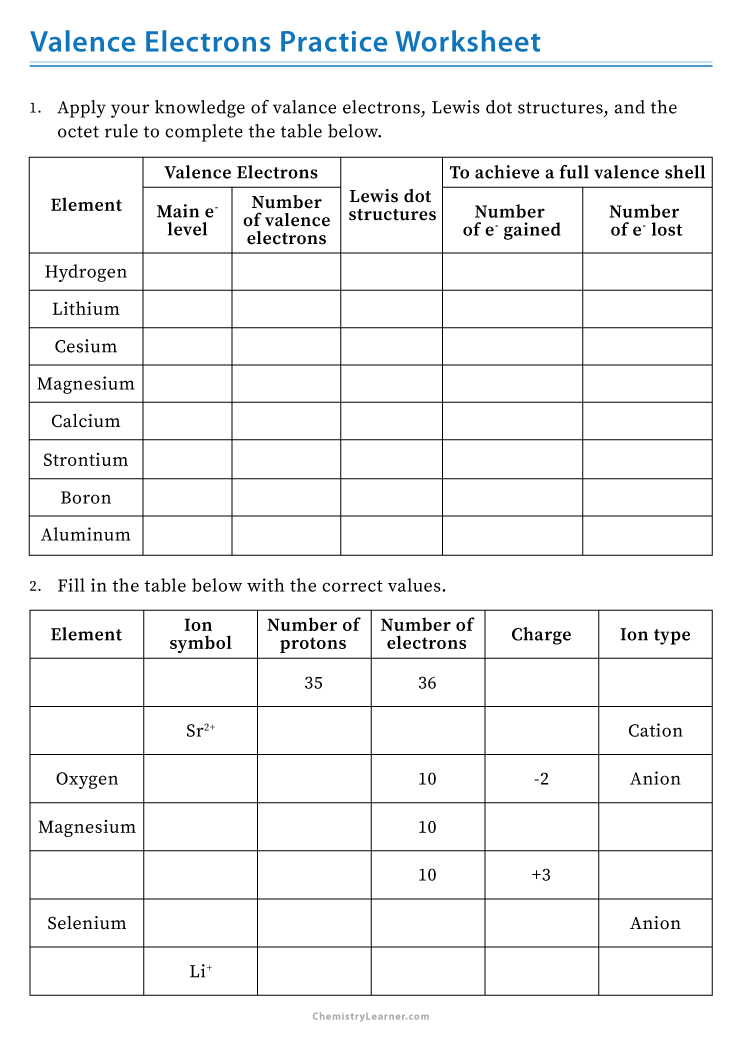
1. Determining Valence Electrons for Atoms

Here are some typical questions you might encounter:
- How many valence electrons does Aluminum (Al) have?
Aluminum is in Group 13 of the periodic table, meaning it has 3 valence electrons.
- What are the valence electrons for Oxygen (O)?
Oxygen is in Group 16, so it has 6 valence electrons.
2. Electron Configuration and Valence Electrons

Electron configuration can help identify the number of valence electrons:
- What is the electron configuration for Lithium (Li), and how many valence electrons does it have?
Lithium has the electron configuration 1s² 2s¹, so it has 1 valence electron.
- Can you find the valence electrons in Silicon (Si) based on its electron configuration?
Silicon’s electron configuration is 1s² 2s² 2p⁶ 3s² 3p². The last shell has 4 electrons, so Silicon has 4 valence electrons.
3. Understanding Lewis Dot Structures
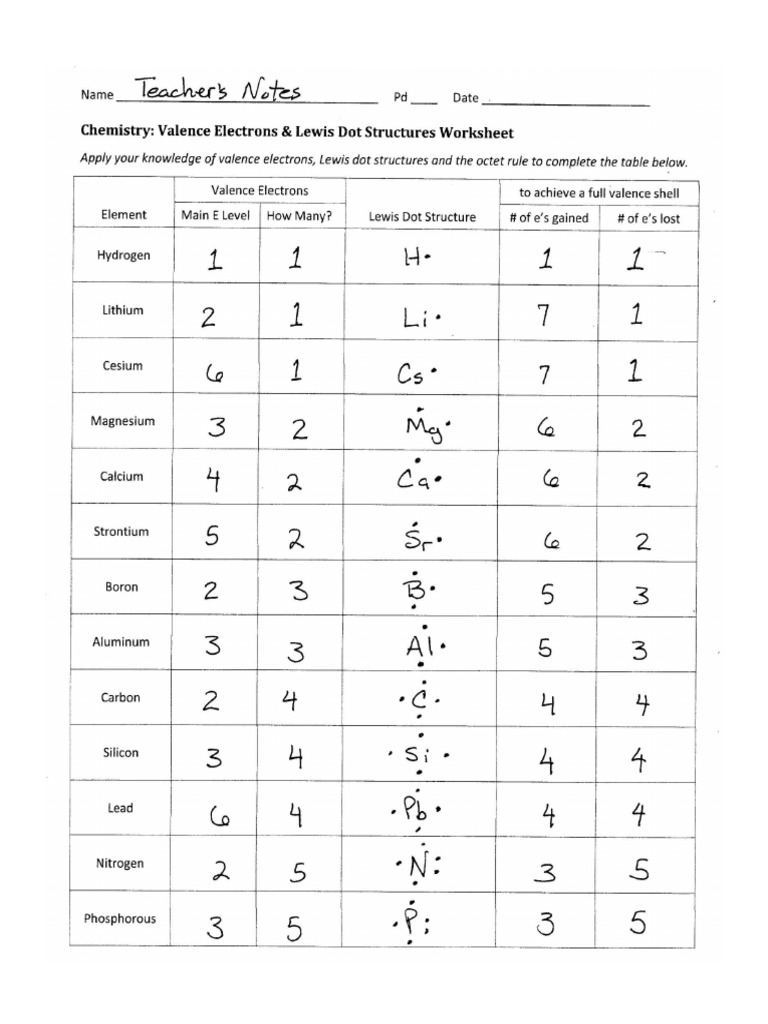
Lewis structures are diagrams used to show valence electrons:
- Draw the Lewis dot structure for Phosphorus (P).
Phosphorus has 5 valence electrons, so the Lewis dot structure would look like:
P: · · · · · · - How does a Lewis structure help in determining the number of valence electrons?
Lewis structures show the valence electrons as dots around the atom, which can be easily counted.
4. Grouping Elements Based on Valence Electrons

Elements in the same group share the same number of valence electrons:
- List elements in Group 2 and their valence electrons.
Elements in Group 2, also known as the Alkaline Earth Metals, have 2 valence electrons. Examples include Beryllium (Be), Magnesium (Mg), and Calcium (Ca).
5. Valence Electrons in Ionic and Covalent Bonding

The number of valence electrons influences the type of bond an atom will form:
- Explain how valence electrons play a role in ionic bonding.
Atoms with few valence electrons (such as metals) tend to lose these electrons, while those with nearly full valence shells (non-metals) gain electrons, resulting in ionic bonds where ions attract each other.
- How do covalent bonds differ in terms of valence electrons?
Covalent bonds occur when atoms share electrons to achieve a stable electron configuration, typically octet or duet configurations.
By working through these valence electrons worksheet answers, you'll enhance your grasp on how atoms behave in chemical environments. Practice will allow you to predict bonding behavior, understand reactivity, and recognize patterns in the periodic table.
Notes:

🔍 Note: Remember that transition metals (in the d-block of the periodic table) can have variable valence electrons due to their complex electron configurations.
🔬 Note: A full octet rule isn’t always applicable, especially for elements like hydrogen and helium which follow the duet rule.
In wrapping up, we've explored various ways to determine and understand valence electrons through worksheet answers. This knowledge is not just theoretical but has practical applications in chemical synthesis, material science, and everyday life phenomena. The periodic trends, bond formation, and overall reactivity of elements are all influenced by the valence electrons, making it a key concept in chemistry that connects many topics together. Practice with these answers can significantly improve your understanding and allow you to approach chemistry with confidence.
Why are valence electrons important in chemistry?

+
Valence electrons determine the reactivity of an atom and its capacity to form bonds, influencing the element’s chemical behavior.
How can I find the valence electrons for an element?

+
Check the element’s group number on the periodic table for main group elements, or review its electron configuration to determine the number of electrons in the outermost shell.
What’s the difference between valence electrons and core electrons?

+
Valence electrons are the outermost electrons that participate in bonding, while core electrons are the inner electrons not involved in bonding.