Valence Electrons and Ions: Chemistry Worksheet Guide

In the world of chemistry, understanding the concepts of valence electrons and ions is fundamental for grasping the basics of chemical reactions and bonding. Whether you're a student, an educator, or simply a curious mind, this detailed guide will take you through the intricate dance of electrons and the formation of ions, offering insights into how these principles apply to everyday chemical reactions.
Understanding Valence Electrons

Valence electrons are the electrons in the outermost shell of an atom. These electrons are pivotal because they participate in the formation of chemical bonds.
- Counting Valence Electrons: For most elements, the group number in the periodic table indicates the number of valence electrons. For example, Group 1 elements have 1 valence electron, Group 2 has 2, and so forth up to Group 18 (the noble gases), which typically have 8.
- Role in Reactivity: The number of valence electrons determines an atom's reactivity. Atoms tend to lose, gain, or share electrons to achieve a stable configuration, often aiming for the electron configuration of the nearest noble gas.
- Energy Levels: Valence electrons occupy the highest energy levels in an atom. The electron configuration provides insights into which energy level these electrons reside.
Consider the following example:
| Element | Valence Electron Configuration |
|---|---|
| Carbon (C) | 2s2 2p2 (4 valence electrons) |
| Oxygen (O) | 2s2 2p4 (6 valence electrons) |
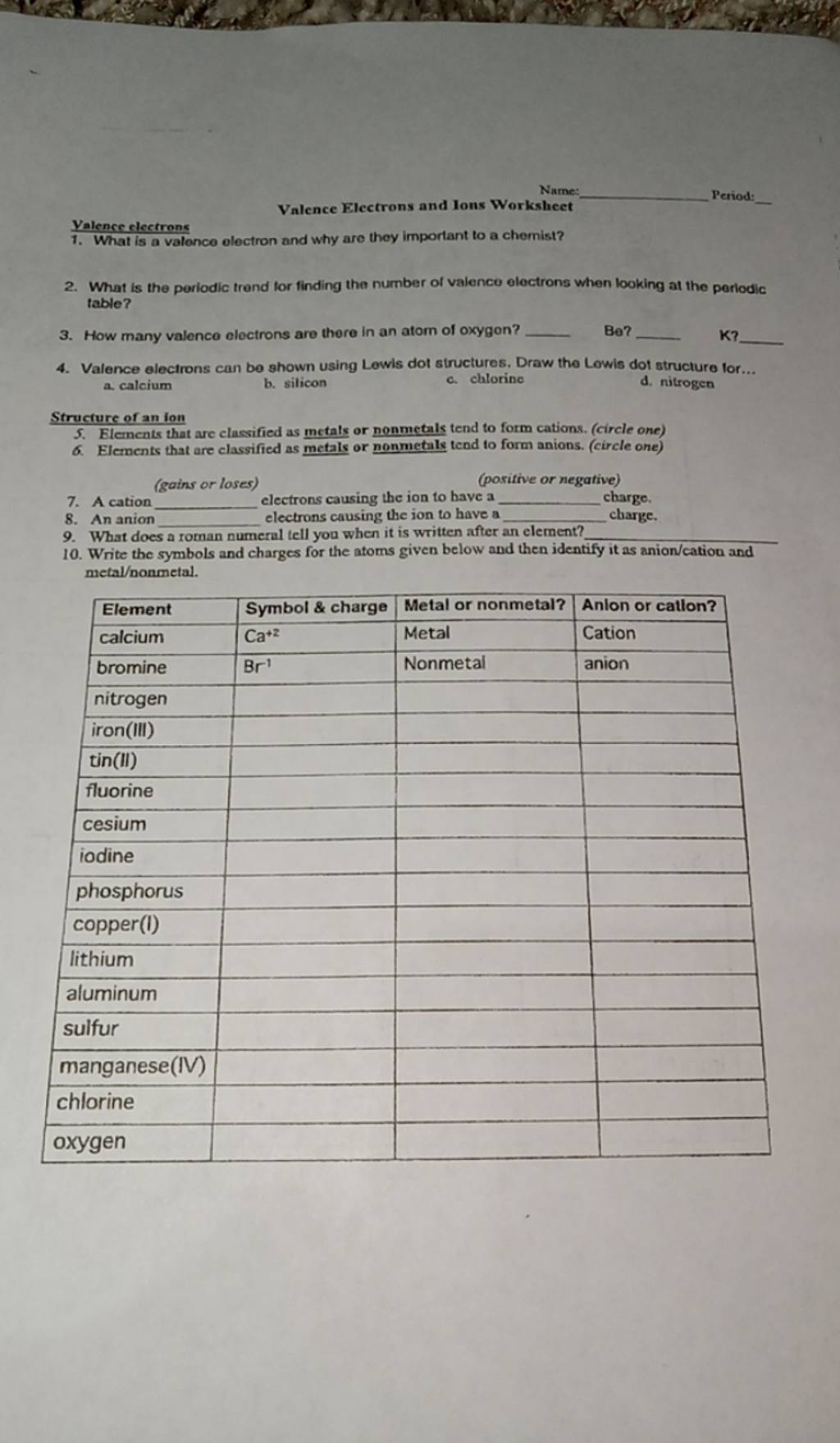
Formation of Ions
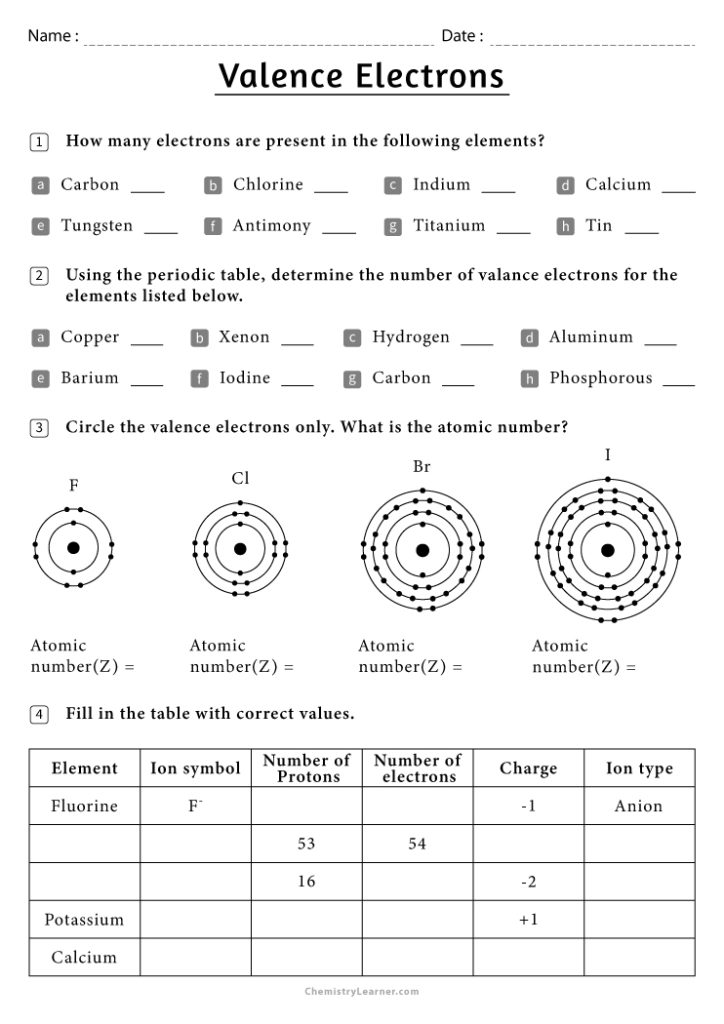
When atoms gain or lose electrons, they become ions. Here's how it happens:
- Positive Ions (Cations): Atoms with fewer than 4 valence electrons tend to lose these electrons to achieve a stable octet, forming positive ions. For instance, sodium (Na) loses one electron to become Na+.
- Negative Ions (Anions): Atoms with more than 4 valence electrons often gain electrons to achieve the stable octet. For example, chlorine (Cl) gains one electron to become Cl-.
Here's a simple depiction:
| Element | Neutral Atom Configuration | Ion Configuration |
|---|---|---|
| Sodium (Na) | [Ne] 3s1 | [Ne] (Na+) |
| Chlorine (Cl) | [Ne] 3s2 3p5 | [Ar] (Cl-) |
Chemical Bonding

Valence electrons play a significant role in the formation of chemical bonds:
- Ionic Bonding: Occurs when one atom donates electrons to another, creating a cation and an anion which are attracted to each other due to opposite charges. An example is the formation of sodium chloride (NaCl).
- Covalent Bonding: Here, atoms share electrons to achieve a stable electron configuration. Water (H2O) is a classic example where hydrogen shares electrons with oxygen.
The way atoms achieve stability through bonding can be complex:
🔬 Note: While the octet rule is a useful guideline, there are exceptions, especially with transition metals and elements in the third row and beyond of the periodic table due to their ability to expand their octets.
Practical Applications

Understanding valence electrons and ion formation is not just academic; it has practical implications:
- Battery Technology: The movement of ions through electrolytes in batteries is essential for energy storage and release.
- Chemical Synthesis: Predicting how molecules will react based on the valence electrons of their constituent atoms helps in synthesizing new compounds.
- Biological Systems: Ion transport across cell membranes is critical for various physiological processes, including nerve impulses and muscle contractions.
The interaction between atoms and ions governs much of the chemistry we experience daily:
Summary of Key Points

Valence electrons are critical in determining an atom's chemical behavior. They dictate how atoms bond to form molecules or ions, influencing reactivity and stability:
- Valence electrons are key to understanding atomic bonding and reactivity.
- Ions form when atoms lose or gain electrons, leading to ionic bonding.
- Covalent bonds share electrons to stabilize atoms.
- These principles underpin the synthesis of compounds and the technology of batteries among other applications.
Why are valence electrons important in chemistry?

+
Valence electrons determine the chemical properties of elements, including their bonding behavior, reactivity, and the types of bonds they can form.
How do ions affect chemical reactions?

+
Ions facilitate ionic reactions where the transfer of electrons leads to electrostatic attractions, influencing the formation of compounds and the speed of reactions.
What are the differences between ionic and covalent bonds?

+
Ionic bonds involve the transfer of electrons, creating charged ions, while covalent bonds involve the sharing of electrons between atoms.
Can valence electrons be more than 8?

+
Yes, but this typically occurs with elements in the third row and beyond of the periodic table. These elements can have more than 8 electrons in their valence shell due to the availability of d-orbitals.
✨ Note: Always consult the most recent data and resources when studying chemistry, as research continually updates our understanding of these phenomena.
Chemistry is a fascinating field where the tiniest particles, like valence electrons, dictate the vast array of substances and reactions we encounter. By understanding valence electrons and ions, we unlock the potential to manipulate matter, creating everything from the medications that heal us to the technologies that connect us. Keep exploring, keep learning, and remember that every atom’s tale is a story of balance and interaction, a fundamental narrative of the universe itself.