Valence Electron Worksheet: Simplified Answer Key

In the world of chemistry, understanding the behavior of atoms involves grasping how their outermost shell, or valence shell, is configured. Valence electrons are particularly important as they are the electrons involved in chemical bonding. This comprehensive guide will walk you through the intricacies of valence electron configuration, highlighting key concepts, offering insights into how to determine the number of valence electrons, and providing you with a valence electron worksheet and its simplified answer key.
What Are Valence Electrons?

Valence electrons are the electrons in the outermost shell of an atom. These electrons are critical in determining how an atom can bond with others. Here's a quick rundown:
- They are involved in the chemical reactions of an atom.
- Their number can indicate an element's reactivity.
- They dictate the atom's electron configurations in chemical bonds.
How to Find Valence Electrons?

To find the number of valence electrons, consider the following steps:
- Identify the Element's Position on the Periodic Table: The periodic table is grouped in blocks according to electron configurations. Look at the group number; for main group elements, this is the same as the number of valence electrons.
- Understand the Electron Shells: The first shell (n=1) can hold up to 2 electrons, while the second and third can hold up to 8, and subsequent shells have higher capacities. Valence electrons are those in the outermost shell.
- Use the Element's Atomic Number: This gives the total number of electrons. Subtract the inner shell electrons from this number to get the number of valence electrons.
🧬 Note: For transition metals, determining valence electrons can be more complex due to the involvement of d-orbitals.
The Importance of Valence Electrons
Valence electrons play a pivotal role in:
- Determining Bonding Capacity: The number of valence electrons determines how many bonds an atom can form.
- Dictating Chemical Properties: Elements within the same group on the periodic table share similar properties due to having the same number of valence electrons.
- Influencing Polarity: Electronegativity, or the atom's ability to attract shared electrons, is influenced by the number of valence electrons.
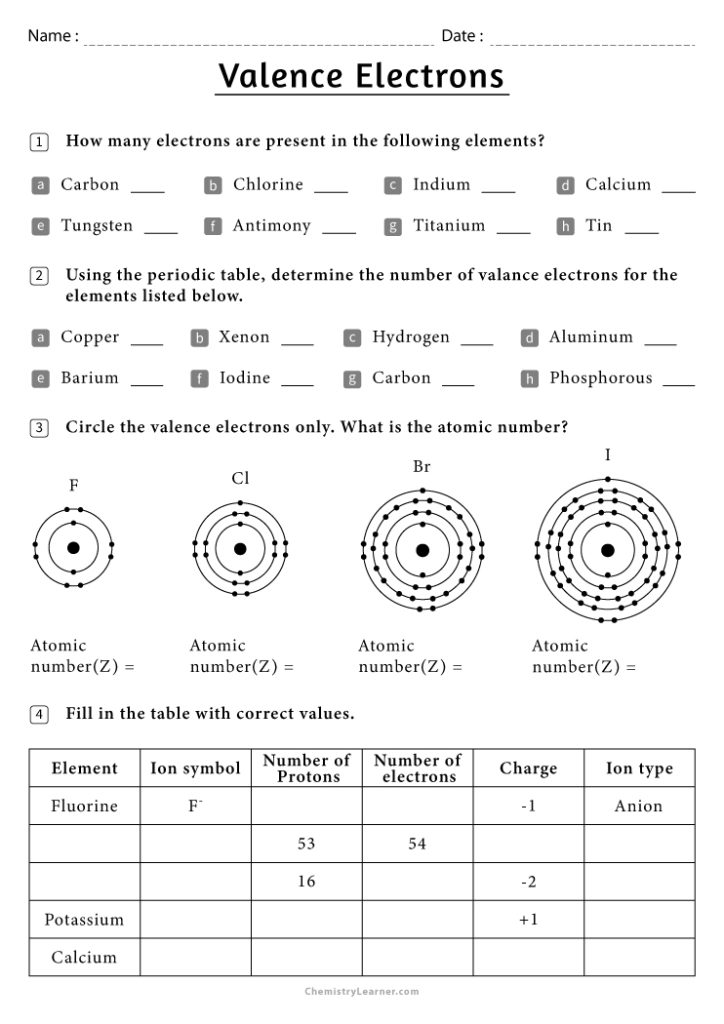
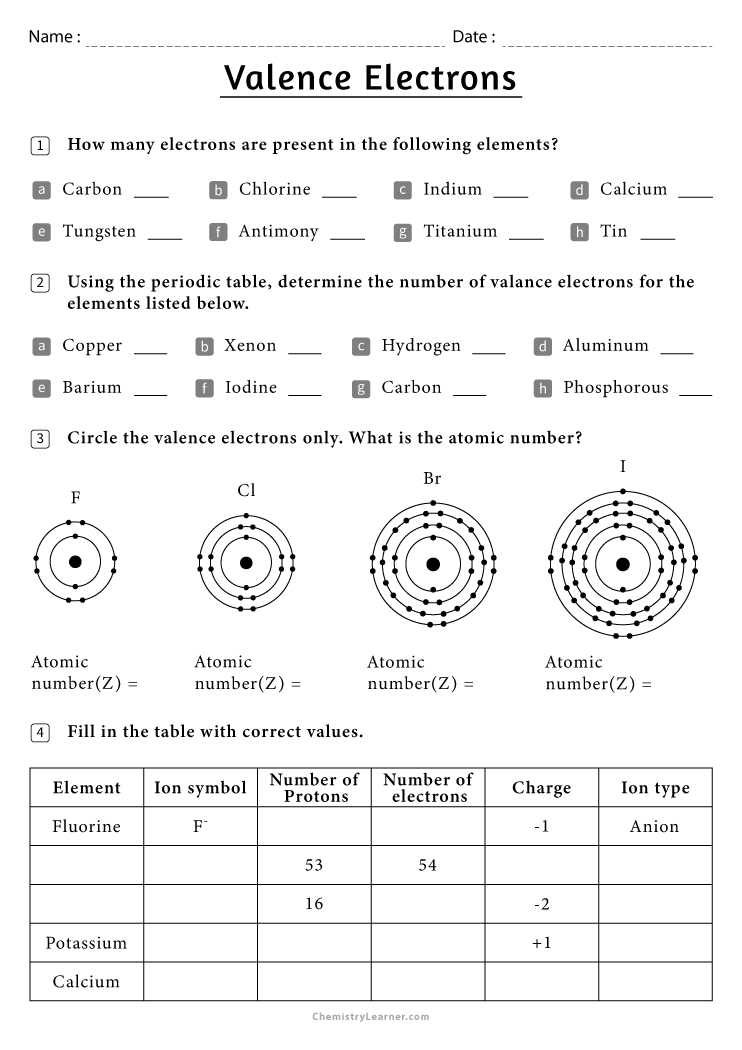
Valence Electron Worksheet

Here's a worksheet to help you practice identifying the number of valence electrons:
| Element | Number of Valence Electrons |
|---|---|
| Hydrogen (H) | |
| Helium (He) | |
| Carbon (C) | |
| Neon (Ne) | |
| Chlorine (Cl) | |
| Potassium (K) |

Below are the simplified answers for the worksheet:
- Hydrogen (H): 1 valence electron
- Helium (He): 2 valence electrons (exception with a full valence shell)
- Carbon (C): 4 valence electrons
- Neon (Ne): 8 valence electrons (noble gas)
- Chlorine (Cl): 7 valence electrons
- Potassium (K): 1 valence electron
The key points to take away from this exploration of valence electrons are their crucial role in chemistry. They influence bonding, reactivity, and many physical and chemical properties of elements. By understanding how to determine the number of valence electrons and using tools like the periodic table, students and chemists can predict and understand the behavior of different elements and compounds.
Why are valence electrons important in chemistry?

+
Valence electrons are involved in the formation of chemical bonds, thereby influencing the reactivity, bond type, and overall chemical properties of an element.
How can you determine the number of valence electrons?
+
By looking at the group number on the periodic table for main group elements or by subtracting inner shell electrons from the atomic number for transition metals.
What is the difference between valence and core electrons?

+
Valence electrons are the electrons in the outermost shell and participate in bonding, while core electrons are the inner shell electrons not involved in chemical reactions.
Do all atoms in the same group have the same number of valence electrons?
+
Yes, elements within the same group on the periodic table share the same number of valence electrons, which is why they have similar chemical properties.
In conclusion, the knowledge of valence electrons not only simplifies the study of chemical reactions but also opens up the understanding of how elements interact to form compounds, showcasing the beauty and complexity of chemistry.