Master Valence Electrons with Our Easy Worksheet
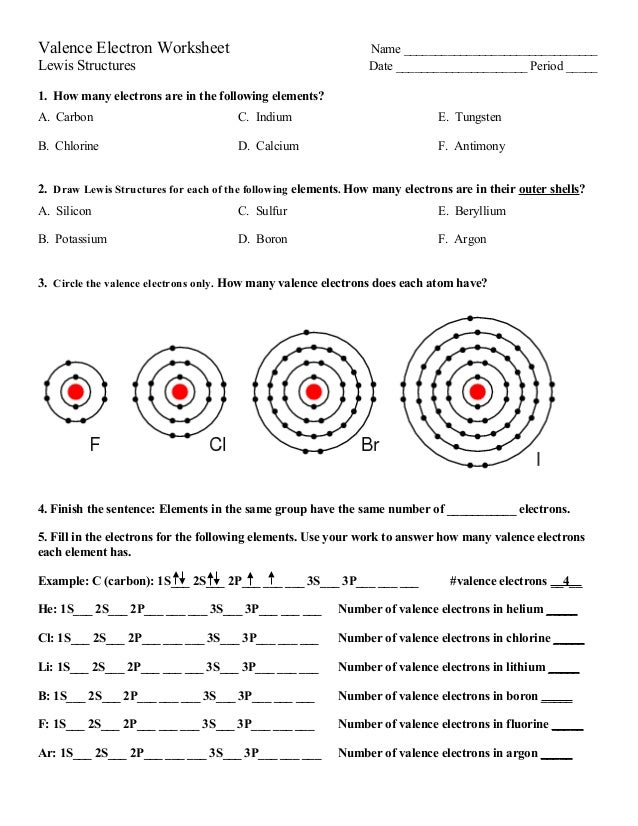
When it comes to understanding chemistry, one cannot underestimate the importance of mastering valence electrons. These electrons, which reside in the outermost shell of an atom, are pivotal for determining how atoms interact, bond, and react with each other. A solid grasp of valence electrons is not only fundamental for students of chemistry but also crucial for anyone seeking to understand the basics of chemical bonding, reactivity, and even molecular geometry. In this extensive guide, we'll delve into valence electrons, offering an easy-to-follow worksheet that helps in demystifying this essential concept.
What are Valence Electrons?
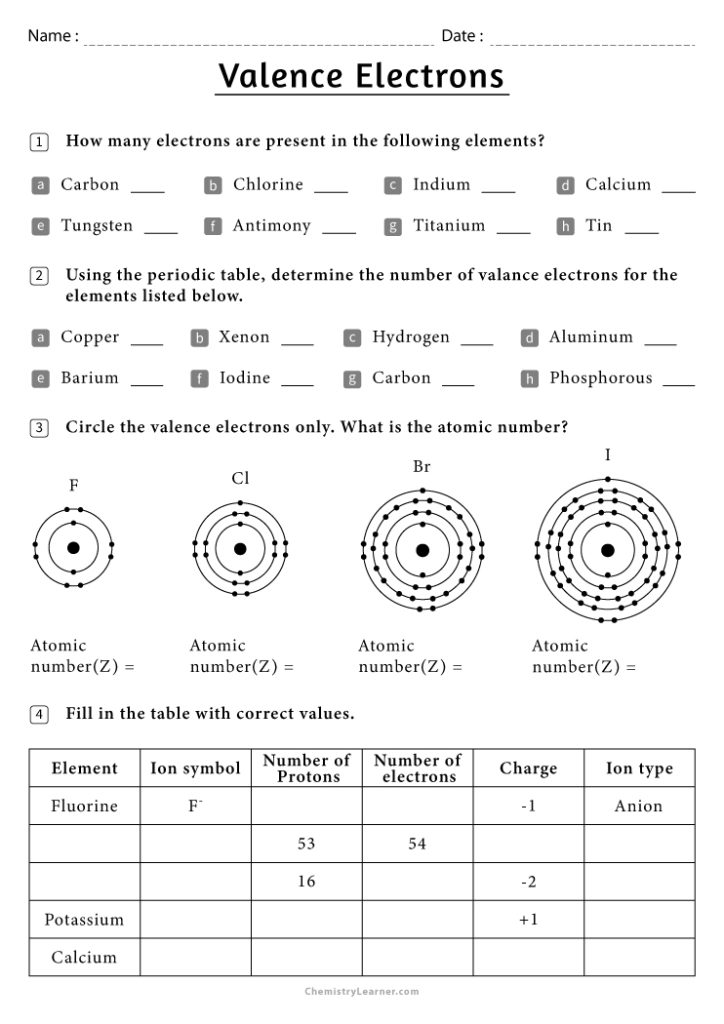

Valence electrons are the electrons in the outermost shell of an atom that are involved in chemical reactions. These electrons determine an atom’s:
- Chemical reactivity: How readily an atom will engage in chemical reactions.
- Bonding behavior: Whether it will form ionic or covalent bonds.
- Element group in the periodic table: Elements with similar valence electron configurations tend to be grouped together.
Identifying Valence Electrons

Here’s a step-by-step guide on how to identify the number of valence electrons:
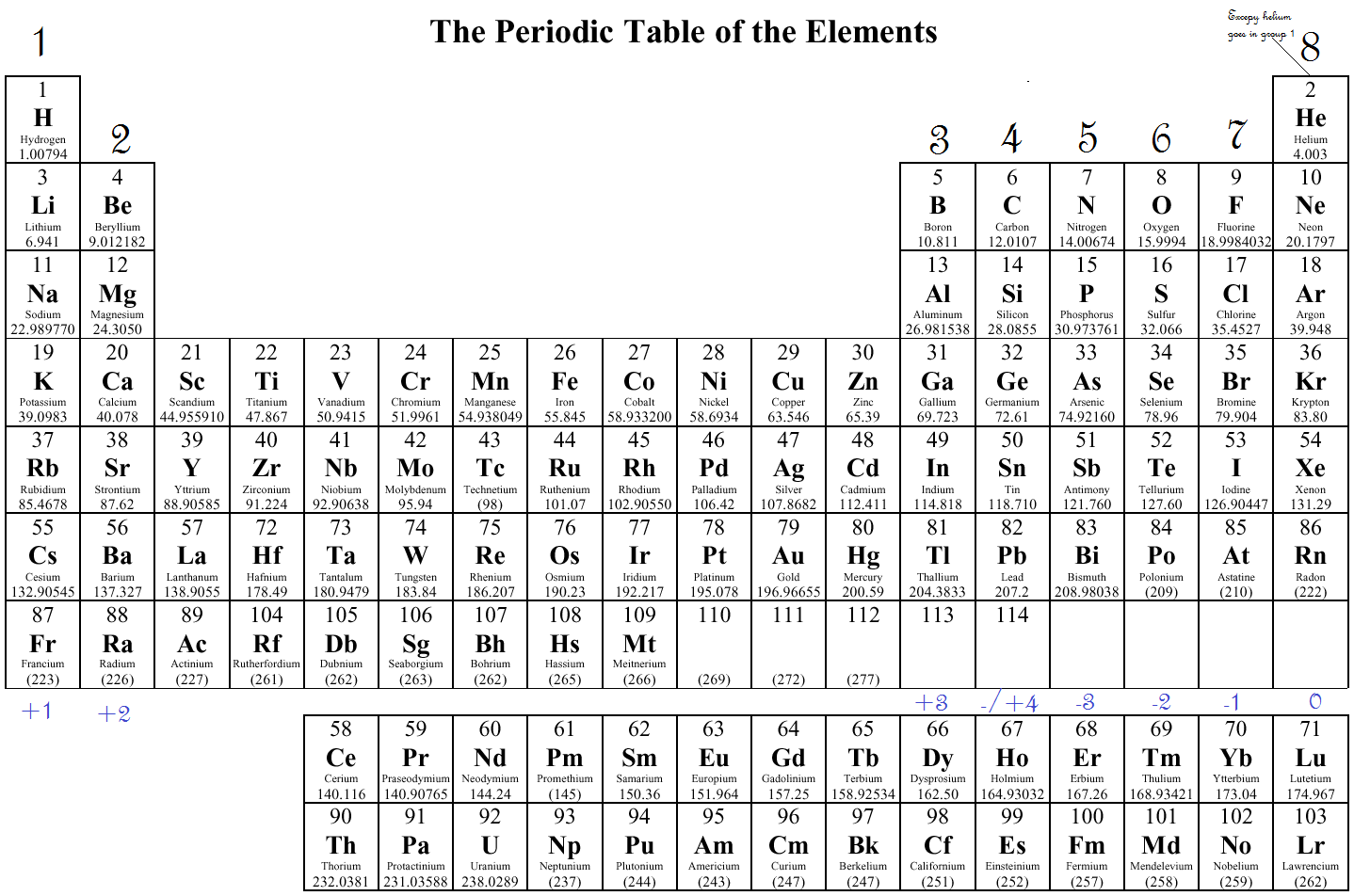
- Determine the atomic number: Find the element’s position on the periodic table.
- Locate the element group: Elements within the same group have the same number of valence electrons.
- Use the periodic table structure:
- For main group elements (Groups 1-2 and 13-18), the group number indicates the number of valence electrons.
- For transition metals, determining valence electrons can be more complex due to variable oxidation states.
🔍 Note: Transition metals often have an unpredictable number of valence electrons since they can form multiple oxidation states.
The Valence Electron Worksheet

| Element | Atomic Number | Group | Valence Electrons |
|---|---|---|---|
| Hydrogen | 1 | 1 | 1 |
| Helium | 2 | 18 | 2 |
| Carbon | 6 | 14 | 4 |
| Oxygen | 8 | 16 | 6 |
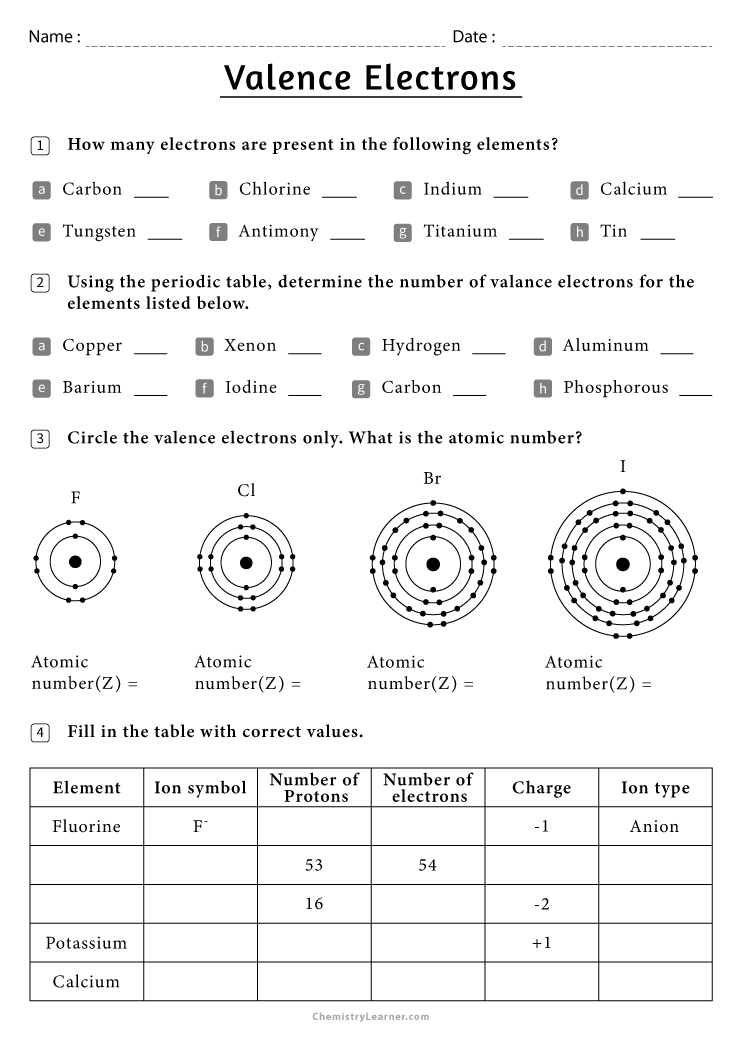
This worksheet serves as an engaging tool to:
- Recognize patterns in valence electron configurations.
- Understand the logic behind atomic interactions.
- Familiarize with the periodic table structure for easier recall.
The Role of Valence Electrons in Chemistry
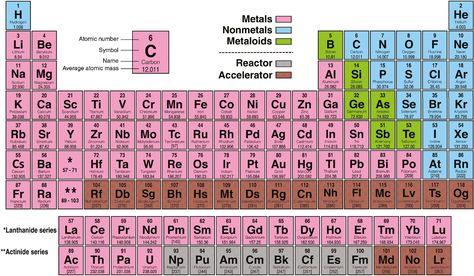
Valence electrons are not just a count; they significantly influence an element’s:
- Reactivity: Elements with full or almost full outer shells are typically less reactive than those with partially filled shells.
- Type of Bonding: Whether the bonds formed will be ionic, covalent, or metallic.
- Octet Rule: Many elements aim to achieve a full octet in their valence shell, which drives chemical reactions.
📖 Note: The octet rule is not universally applicable. Some elements like Hydrogen prefer two electrons, while noble gases already have full valence shells.
Advanced Concepts: Electron Configuration and Oxidation States

Understanding electron configurations deepens our comprehension of valence electrons:
- Electron Configuration: Shows the distribution of electrons among atomic orbitals.
- Valence Electrons in Electron Configuration: Valence electrons are those in the highest energy levels.
Additionally, the concept of oxidation states is intricately linked with valence electrons. Here’s how:
- Elements can lose, gain, or share valence electrons, altering their charge or oxidation state.
- Multiple oxidation states can exist for the same element, particularly in transition metals.
In Closing

To conclude, valence electrons play a vital role in chemical reactions and bonding. By working through the provided worksheet and understanding the principles behind valence electrons, one can gain a deeper insight into how atoms interact. This knowledge is not only useful for academic purposes but also for understanding the world around us at a molecular level. From explaining why metals conduct electricity to understanding the stability of compounds, valence electrons are key to unlocking the mysteries of chemistry.
How do I find the number of valence electrons in an atom?
+
To find the number of valence electrons, look at the element’s group number on the periodic table. For main group elements, the group number equals the number of valence electrons.
Why are valence electrons important?
+
Valence electrons determine an element’s reactivity, the type of chemical bonds it can form, and its placement in the periodic table. They are crucial for understanding chemical reactions and bonding.
What does it mean when an element has a full set of valence electrons?
+
An element with a full valence shell, typically 8 electrons, is very stable and less reactive, following the octet rule. This stability makes noble gases inert.
Can an element have different numbers of valence electrons?
+
Yes, particularly in transition metals, which can exhibit multiple oxidation states due to the versatility in their valence electron configurations.
How do valence electrons affect the type of bond an atom forms?

+
The number and arrangement of valence electrons dictate whether an atom will lose, gain, or share electrons to achieve stability, leading to ionic, covalent, or metallic bonds.