5 Essential Answers for Atomic Structure Worksheets

Understanding atomic structure is foundational for grasping numerous chemical principles. Atomic structure worksheets are common tools used in science education to help students conceptualize atoms, their constituents, and how they interact. Here, we delve into five essential answers that frequently appear in atomic structure worksheets, elucidating the critical aspects of atomic theory and chemistry.
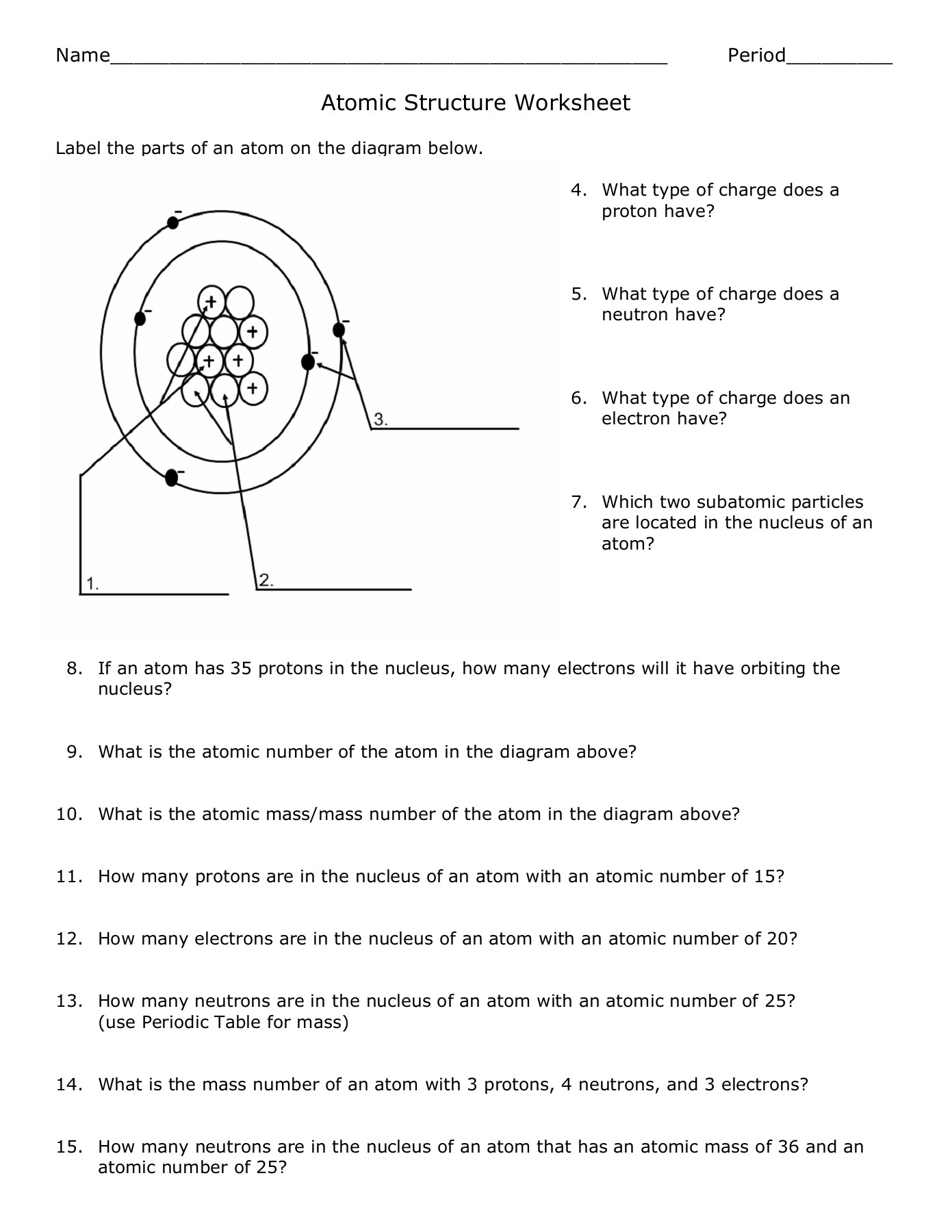
1. What Are the Basic Components of an Atom?


Atoms, the smallest unit of an element, consist of three primary components:
- Protons: Positively charged particles found in the nucleus, providing an element's identity by their number.
- Electrons: Negatively charged particles that orbit the nucleus in shells or energy levels, involved in bonding and chemical reactions.
- Neutrons: Neutral particles in the nucleus, contributing to the mass of the atom but not its charge.
🔬 Note: While quarks are the fundamental building blocks of protons and neutrons, they are typically not introduced until advanced studies due to their complexity.
2. How Do We Identify an Element?

Each element is uniquely identified by:
- Atomic Number: The number of protons, which defines the element. For instance, hydrogen has an atomic number of 1, with one proton, making it different from all other elements.
- Chemical Symbol: A shorthand notation, like ‘H’ for hydrogen, derived from its Latin or English name.
- Mass Number: The total number of protons and neutrons in the nucleus.
3. What is an Electron Shell or Energy Level?


Electrons occupy specific energy levels around the nucleus, known as shells or energy levels. Here's how they are organized:
- Principal Quantum Number (n): Each shell is designated by an integer (1, 2, 3, etc.), with higher numbers indicating shells further from the nucleus.
- Electron Capacity: The first shell holds up to 2 electrons, the second up to 8, and subsequent shells follow the formula 2n2.
- Valence Electrons: Electrons in the outermost shell that participate in chemical bonding.
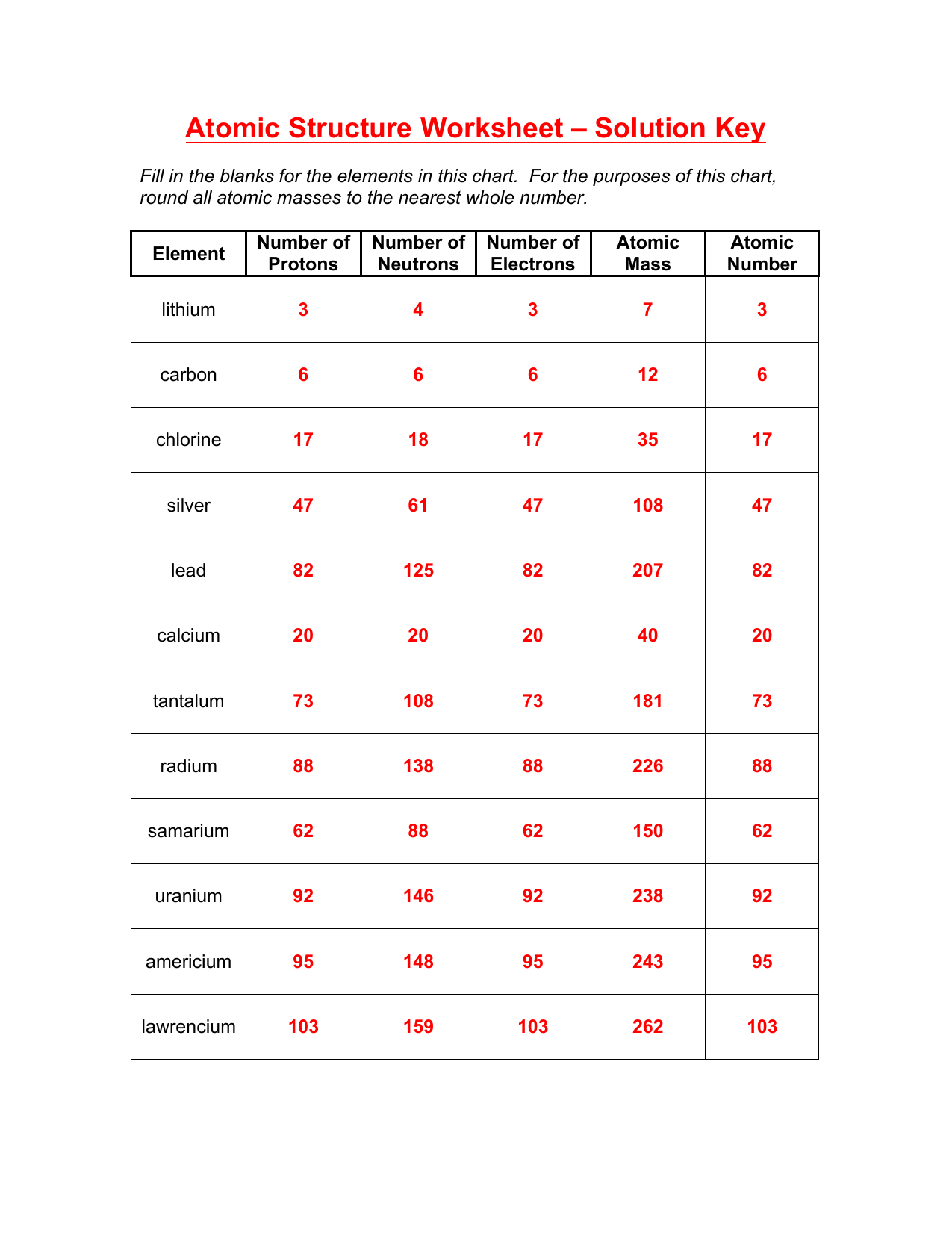
4. How Do Isotopes Fit into Atomic Structure?

Isotopes are atoms of the same element with different numbers of neutrons. This leads to:
- Same Atomic Number: Isotopes share the same proton count and thus element identity.
- Different Mass Number: Isotopes vary in mass due to differing neutron counts.
- Common Isotopes: Elements like carbon have common isotopes like Carbon-12 and Carbon-14.
| Isotope | Protons | Neutrons | Electrons |
|---|---|---|---|
| Carbon-12 | 6 | 6 | 6 |
| Carbon-13 | 6 | 7 | 6 |
| Carbon-14 | 6 | 8 | 6 |
🔬 Note: Isotopes can be stable or radioactive, with radioactive isotopes decaying over time to become stable isotopes or different elements.
5. What is the Role of Atomic Orbitals?


Atomic orbitals describe the probable location of electrons within an atom:
- s, p, d, f: These letters represent different shapes and energy levels of electron clouds or probability distributions.
- Electron Spin: Electrons in orbitals have either an up or down spin, influencing their pairing.
- Hund's Rule: Electrons fill orbitals singly before pairing, promoting the lowest energy state.
Understanding atomic structure through these five questions provides a solid foundation for students to explore the fascinating world of chemistry. From visualizing atomic models to understanding how elements differ due to isotopic variations, these concepts are crucial for appreciating both the simplicity and complexity of the microscopic world.
Having explored the core components of an atom, their arrangement, and how they differ across elements, we come to appreciate the beauty of nature's design at the atomic level. Each element's unique behavior and chemical properties stem from these foundational principles, opening doors to further exploration in chemistry and physics.
Why are electron shells important?

+
Electrons in different shells have varying energies, which dictates the atom’s reactivity and bonding behavior. Valence electrons, in particular, determine how atoms will interact with each other.
How can we determine if an isotope is stable?

+
The stability of isotopes is often determined by the neutron-to-proton ratio. If this ratio falls outside certain limits, the isotope may be unstable and undergo radioactive decay to reach stability.
What’s the difference between an orbital and an orbit?
+
An orbit refers to the Bohr model’s fixed path of electrons around the nucleus. An orbital represents a region of space where there’s a high probability of finding an electron, reflecting modern quantum mechanics.