5 Essential Tips for Understanding Mole Relationships in Chemistry

Understanding mole relationships in chemistry is pivotal for students and professionals alike who delve into the realm of chemical reactions, stoichiometry, and quantitative analysis. The concept of moles helps to bridge the gap between the microscopic world of atoms and molecules and the macroscopic world we can measure and observe. Here, we explore five essential tips that can significantly enhance your comprehension of mole relationships in chemistry.
Understanding the Concept of Mole

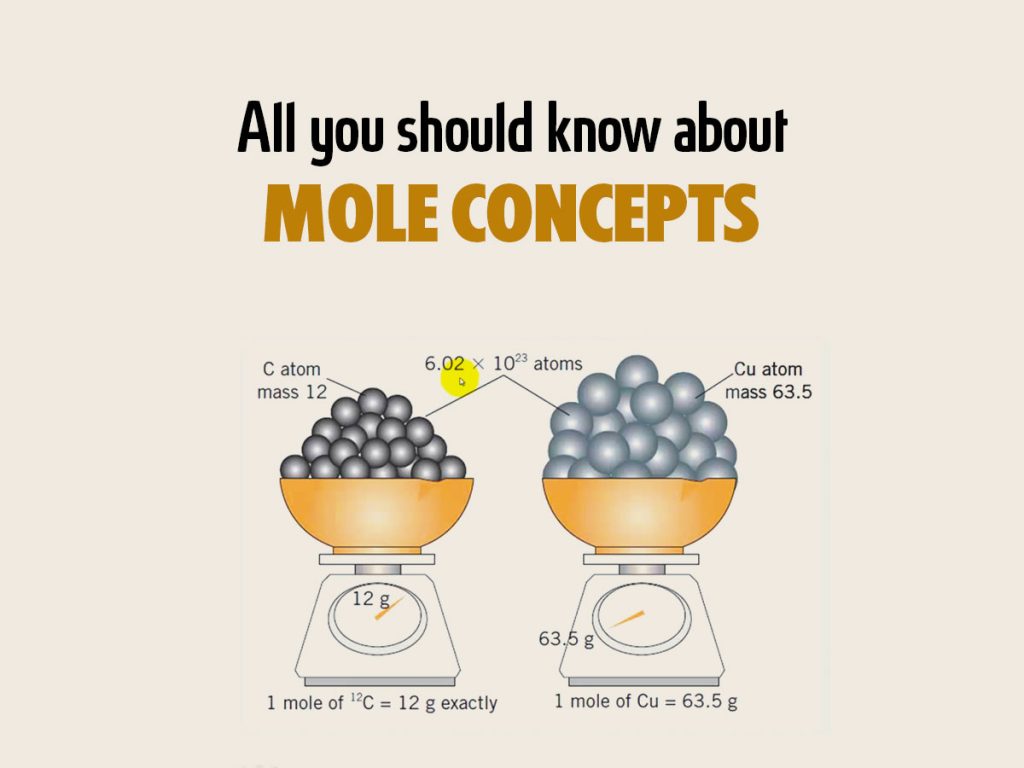
The mole is a unit of measure that defines the number of entities, much like a dozen defines 12 items. In chemistry, a mole is Avogadro’s number (approximately 6.022 x 1023) of entities, be it atoms, molecules, ions, or other particles. Here’s how to understand this concept:
- Counting in chemistry: While we cannot count atoms individually, moles provide a method to quantify them.
- Equivalence: A mole of any substance contains the same number of particles, allowing for direct comparison.
📘 Note: Remember that Avogadro’s number is a constant, meaning it remains the same regardless of the substance.
Relating Moles to Mass

To connect moles to practical measurements, we need to understand how moles relate to the mass of a substance. Here are the steps:
- Find the molar mass of the substance by summing the atomic masses of its constituent elements.
- Use the molar mass to convert moles to grams or vice versa through the formula:
- Mass (g) = Moles x Molar Mass (g/mol)
Example:
| Substance | Molar Mass (g/mol) | 1 Mole (g) |
| H2O | 18.01528 | 18.01528 |
| NaCl | 58.44 | 58.44 |

🔬 Note: Always double-check calculations involving molar masses, as even small errors can lead to significant discrepancies.
Stoichiometry and Chemical Equations

Stoichiometry is the study of quantitative relationships in chemical reactions. Here’s how you can use moles in this context:
- Balance the chemical equation to determine the mole ratios of reactants and products.
- Convert mass or volume of reactants/products to moles.
- Use the mole ratio from the balanced equation to find the moles of other substances involved.
Limiting Reactant and Percent Yield

In most real-world reactions, not all reactants are used up simultaneously. Here’s how to deal with this:
- Determine the limiting reactant by comparing the moles of each reactant with the stoichiometric coefficients.
- Calculate the theoretical yield using the limiting reactant.
- Measure the actual yield to calculate the percent yield, which provides insight into the efficiency of the reaction:
Percent Yield = (Actual Yield / Theoretical Yield) x 100%
Practical Applications in Real-Life Chemistry

Understanding mole relationships goes beyond academia; it has numerous practical applications:
- Drug Formulation: Accurate mole calculation ensures the right dosage in pharmaceuticals.
- Food Industry: Control of nutrients, preservatives, or flavors in food products.
- Environmental Analysis: Measuring pollutant concentrations or emissions in air and water.
The deep understanding of mole relationships not only facilitates better lab work but also enables us to appreciate the complexity and precision required in various industries. By mastering these principles, one can unlock the quantitative side of chemical reactions, leading to innovative solutions in science, health, and beyond.
Why is the mole concept important in chemistry?

+
The mole concept provides a bridge between the microscopic scale of atoms and molecules and the macroscopic scale we can measure, making it fundamental for all chemical calculations and reactions.
How do you calculate the molar mass of a compound?

+
Molar mass is calculated by summing the atomic masses of all elements in a compound, which can be found on the periodic table.
What is stoichiometry and why is it used?

+
Stoichiometry is the quantitative study of reactants and products in chemical reactions, allowing chemists to predict how much product will form from given reactants.
What is the difference between theoretical and actual yield?

+
Theoretical yield is the maximum amount of product that could be formed, whereas the actual yield is the quantity of product obtained in reality, which is often less due to inefficiencies in the reaction or side reactions.
Can moles be used in real-life scenarios outside the lab?
+
Absolutely! Moles are crucial in pharmacology for drug formulation, in nutrition for controlling nutrient intake, and in environmental science for measuring concentrations of pollutants.