Unit 3 Chemistry Worksheet 2 Answer Key Revealed
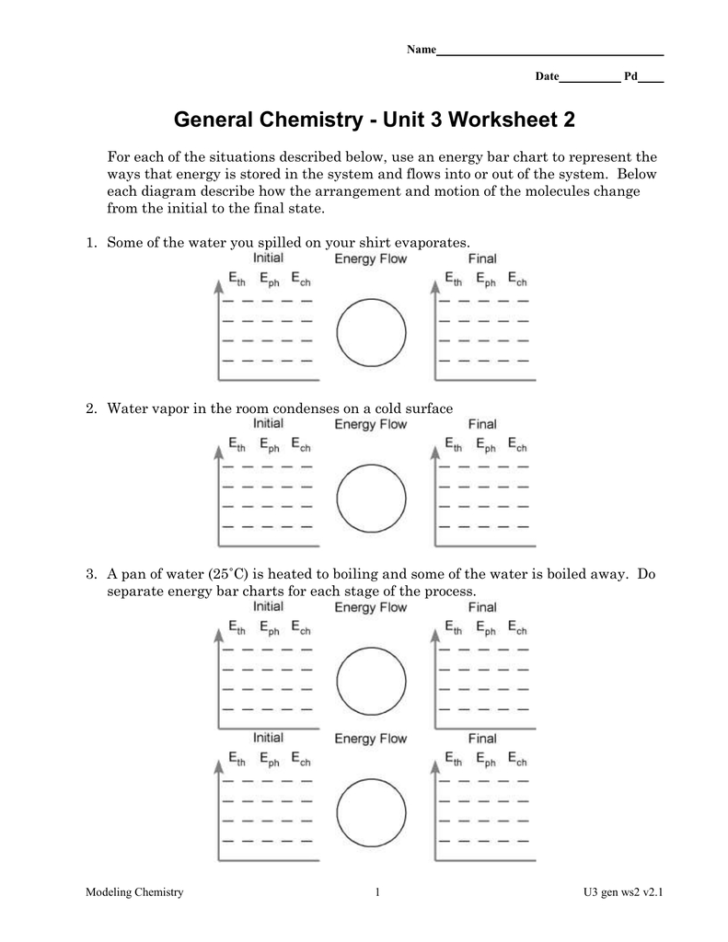
Introduction to Unit 3 Chemistry Worksheet

Unit 3 of your chemistry class likely revolves around topics like thermodynamics, gas laws, and chemical reactions. Worksheets play a crucial role in reinforcing the understanding of these concepts. Today, we’ll be exploring the Unit 3 Chemistry Worksheet 2 and its answer key to help you grasp the content better.
Understanding the Key Concepts
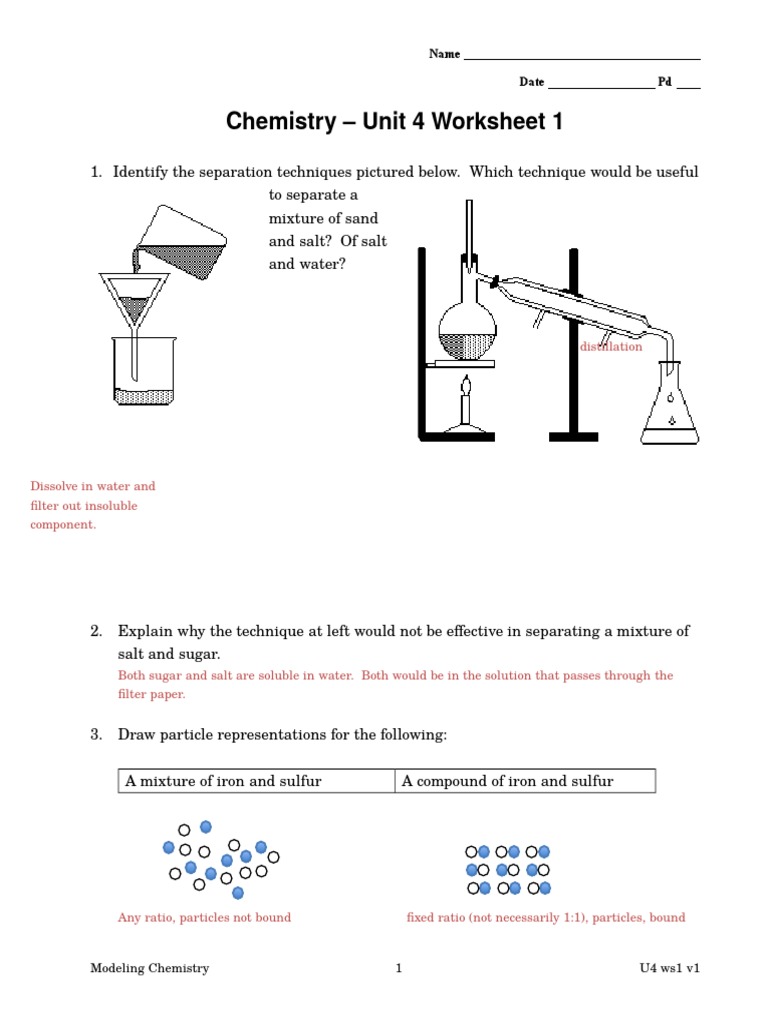
Before delving into the worksheet answers, let’s briefly cover the key areas typically covered in Unit 3:
- Thermodynamics: Understanding heat, work, energy transfer, and system changes.
- Gas Laws: Boyle’s, Charles’, Gay-Lussac’s, and Avogadro’s Law.
- Chemical Reactions: Types of reactions, balancing equations, and stoichiometry.
🔬 Note: If you’re having trouble with any of these concepts, remember that the worksheet is designed to solidify your understanding through practical problems.
Analyzing Worksheet 2

Here’s a breakdown of what’s often covered in a typical Unit 3 Chemistry Worksheet 2:
Questions on Thermodynamics
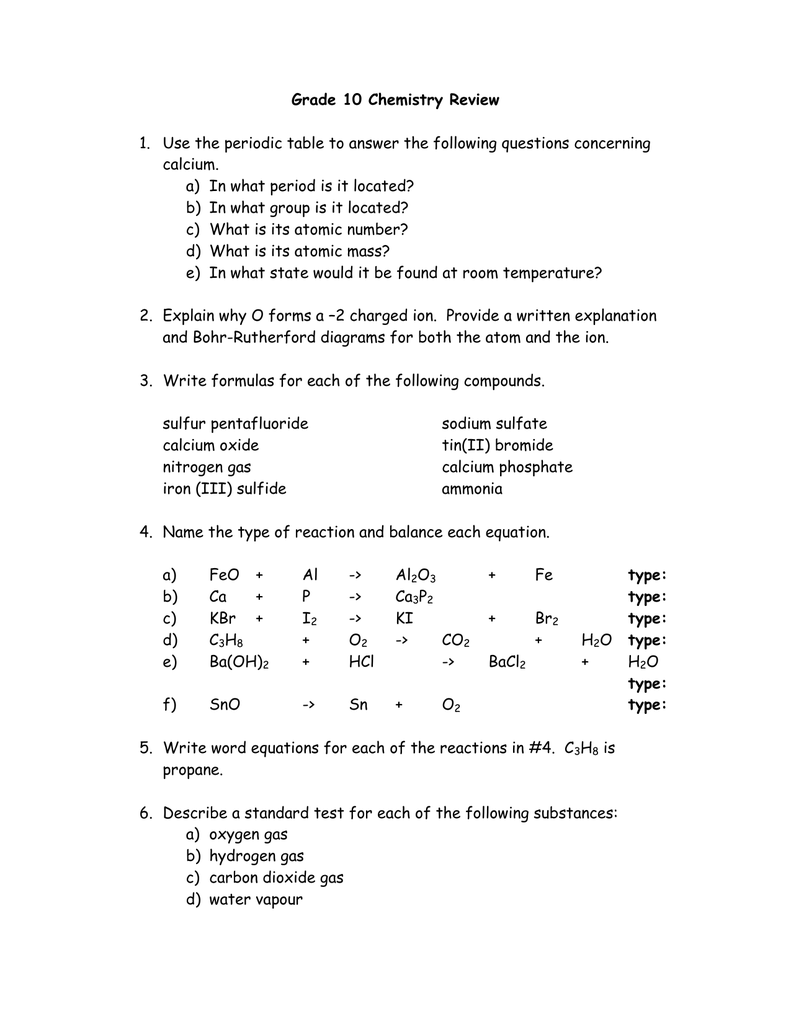
You’ll likely find questions related to:
- Calculating work done by a system or environment.
- Determining changes in enthalpy, entropy, and Gibbs free energy.
- Analyzing the feasibility of reactions.
Questions on Gas Laws
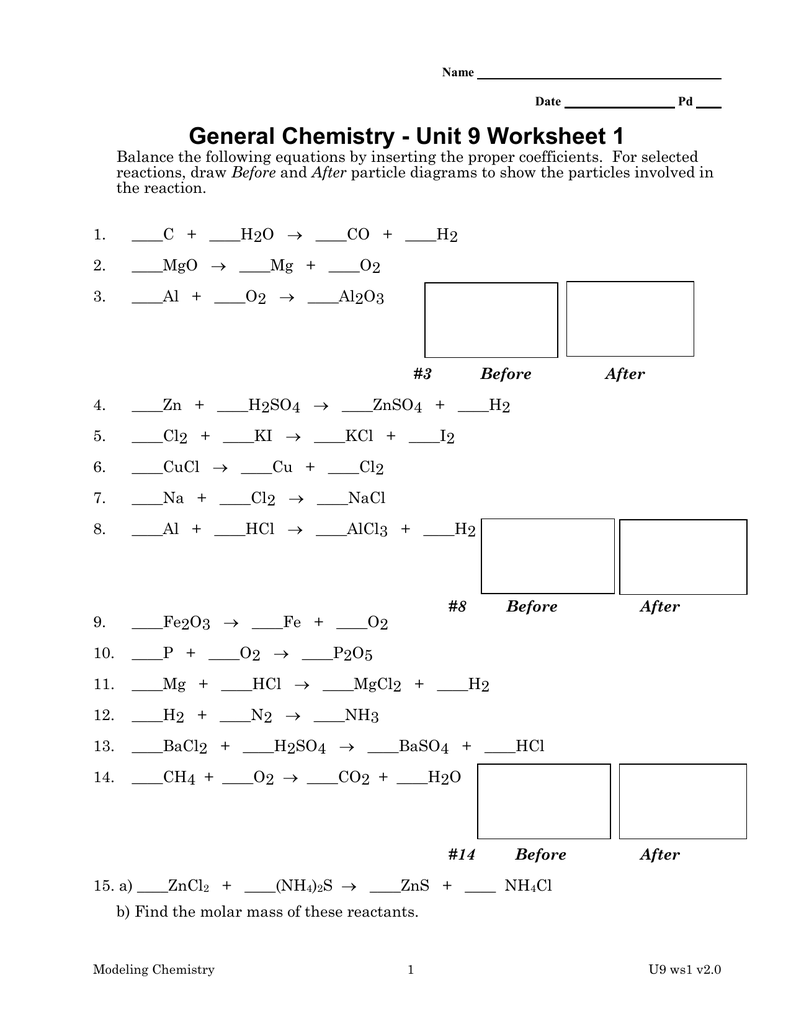
Expect problems that involve:
- Conversions between different gas laws.
- Problems where the relationship between pressure, volume, and temperature is tested.
Chemical Reactions

This section would include:
- Balancing chemical equations.
- Reaction types like precipitation, combustion, acid-base, and redox.
- Stoichiometry problems, which could involve yield calculations, limiting reagents, etc.
Answer Key Insights

While every school might have different versions of the worksheet, here’s a general overview of how you might find answers:
Thermodynamics Problems

Here are some common types of answers:
- Work Calculations: For example, if asked to calculate work done when gas expands against a constant external pressure, you might see answers like “W = -PΔV” with the value calculated in joules or liters-atmospheres.
- Enthalpy Changes: Often, you’ll use Hess’s Law or other methods to determine enthalpy changes, and the answer might be, “ΔH = -120 kJ” or similar.
Gas Law Calculations

Here are common answers to gas law problems:
- For Boyle’s Law, an answer might look like, “P2 = (P1V1) / V2” with the calculated pressure.
- For combined gas law, answers involve plugging in known variables and solving for the unknown.
Chemical Equations and Stoichiometry

Answers here often involve:
- Balancing: The equation is balanced, and the answer might show something like “2Fe + O2 → 2FeO”.
- Stoichiometry: Answers could be “The theoretical yield of H2O is 36g.”
How to Use the Answer Key

Don’t just look for the right answer; use the answer key to:
- Understand the steps to reach the solution.
- Identify common mistakes or misconceptions you might have had.
- Revisit the underlying concepts if you got any question wrong.
⚗️ Note: While having an answer key is helpful, true learning comes from understanding the 'how' and 'why' behind each answer.
Final Thoughts

Working through the Unit 3 Chemistry Worksheet 2 and its answer key isn’t just about finding the correct answers. It’s an opportunity to see the application of concepts in practical scenarios, to recognize patterns, and to enhance your problem-solving skills. Remember, chemistry is about understanding the principles, not merely memorizing facts. By dissecting the problems in the worksheet, you get to see where theory meets application, which is crucial for any science student.
What if my worksheet looks different from the answer key?

+
Worksheets can vary slightly from school to school. Use the answer key to understand the underlying principles rather than focusing solely on matching the exact problem.
Can I use these answers for exams?

+
Memorizing answers without understanding won’t help on exams. Use the answers to understand the logic behind each problem, as tests often include new scenarios where you’ll need to apply learned principles.
How do I get better at stoichiometry?
+
Practice is key. Work through as many stoichiometry problems as possible, focusing on the method of solving rather than just the final answer.